Clinical guidelines recommend the use of antidepressants for moderate to severe adolescent depression (
1). However, even under the controlled conditions of clinical trials, only around 60% of depressed adolescents respond to an initial trial with a selective serotonin reuptake inhibitor (SSRI) (
2). The high rate of nonresponse and the occurrence of suicidal events in depressed adolescents are both important clinical concerns (
2). In this context, efforts to personalize treatment by identifying which patients are most likely to respond to antidepressants and least likely to experience a suicidal event are of high public health significance. Pharmacogenetics, which examines the relationship between genetic variation and clinical response to a given medication, holds promise for personalizing and optimizing pharmacotherapy.
Pharmacogenetic predictors of antidepressant response have primarily focused on the impact of genetic variations in the serotonin transporter promoter gene (SLC64A) and on other serotonin-related receptors because of the mechanisms of action of SSRIs (
3). Antidepressant treatment response has been associated with the less functional variant of the serotonin transporter promoter gene, polymorphisms in other serotonergic-related receptors (HTR1A and HTR2A), and the serotonin synthesis enzymes tryptophan hydroxylase 1 and 2 (TPH1 and TPH2) (
3–
7). Genes related to noradrenergic neurotransmission (norepinephrine transporter promoter [NET]), glutamatergic neurotransmission (glutamate receptor, ionotropic, kainate 4 [GRIK4]), monoamine metabolism [MAOA], and the hypothalamic-pituitary-adrenal (HPA) axis (corticotropin-releasing hormone receptors [CRHR1], FK506-binding protein 5 [FKBP5]) have also been associated with treatment response (
8–
13). Reported pharmacogenetic predictors of overall response burden and treatment-emergent suicidal events include the serotonin transporter, HTR2A, glutamatergic receptors (glutamate receptor, ionotropic, kainite 2 [GRIK2]; glutamate receptor, ionotropic, AMPA 3 [GRIA3]), and cyclic adenosine monophosphate response element-binding protein [CREB1]) (
1417).
Despite progress in studies on the pharmacogenetics of antidepressant response, only one published study has focused on adolescents (
5). The results showed that the antidepressant effect of citalopram was more vigorous in individuals who were homozygous for the higher transcribing allele of the serotonin transporter gene.
We report on the relationship of polymorphisms in serotonergic and other neurotransmitter systems, as well as those involved in the HPA axis, to both treatment response and the occurrence of suicidal events in the Treatment of SSRI-Resistant Depression in Adolescents study (TORDIA) (
18). In TORDIA, 334 depressed adolescents who had not responded to an adequate trial with an SSRI were randomly assigned to one of four treatments—switch to another SSRI (either fluoxetine, paroxetine, or citalopram), switch to venlafaxine, switch to another SSRI plus cognitive-behavioral therapy (CBT), or switch to venlafaxine plus CBT. By the end of 12 weeks of treatment, the addition of CBT resulted in a higher response rate than the switch to medication alone, but there was no difference in response rate between the two medication groups. Also, there were no differential treatment effects on the occurrence of suicidal events (
18,
19).
On the basis of the above-noted review of the literature, we identified candidate genes that have been reported to be associated with either treatment response or suicidal events. We also investigated polymorphisms in genes that have been reported to be involved in the etiology of depression (brain-derived neurotrophic factor [BDNF] and dopamine transporter [DAT]) or in the mechanisms of action of SSRIs or venlafaxine (NET1 and beta-1 adrenergic receptor [ADRB1]). We examined the relationship between polymorphisms in these genes and both clinical response and the occurrence of suicidal events while controlling for the effects of treatment and other clinical predictors of these two outcomes.
Method
The study methods are described in more detail in our initial report (
18). Recruitment for the study was conducted at six sites. Participants were adolescents 12–18 years of age in active treatment for DSM-IV major depressive disorder (
20), had clinically significant depression as defined by a score ≥40 on the Children's Depression Rating Scale–Revised (
21), and a score ≥4 (moderately severe) on the severity subscale of the Clinical Global Impressions scale (CGI-S) (
22). Participants had received at least 8 weeks of treatment with an SSRI, with at least the last 4 weeks at a dose equivalent to 40 mg of fluoxetine. Exclusion criteria included abnormal laboratory tests, a positive drug screen, two or more adequate trials with an SSRI, prior treatment with venlafaxine or CBT, bipolar spectrum disorder, psychosis, obsessive-compulsive disorder, substance abuse or dependence, autism, eating disorder, hypertension, and, for female participants, pregnancy, nursing an infant, or having unprotected sex.
The TORDIA study and the pharmacogenetics protocol were approved by each site's institutional review board, and separate informed assent/consent was obtained for the treatment and the pharmacogenetic studies. Five of the six sites elected to participate in the pharmacogenetic study, and 176 of a possible 292 patients participated.
Primary Outcome Measures
The two outcome measures for this pharmacogenetic study were adequate clinical response and suicidal events. Adequate clinical response after 12 weeks of treatment was defined a priori as a rating of much or very much improved on the improvement subscale of the Clinical Global Impressions scale (CGI-I) (
22), a decrease >50% in the Children's Depression Rating Scale–Revised score, and a score <40 on the Children's Depression Rating Scale–Revised. Suicidal events were classified according to the Columbia Classification Algorithm of Suicide Assessment using the Brief Suicide Severity Rating Scale (
23), which rates both ideation and behavior on a scale of 0 to 5. For ideation, 0 corresponds to no ideation and 5 to ideation with intent and a plan; for behavior, 0 corresponds to no behavior and 5 to multiple attempts. A suicidal event was defined as new-onset or worsened suicidal ideation or suicidal behavior requiring at least a 1-point increase from baseline on the Brief Suicide Severity Rating Scale. A suicide attempt was defined as "self-injurious behavior with explicit or implied intent to die" (
23). All adverse events were discussed during weekly conference calls and were classified by consensus. In addition, interrater reliability in the assessment of suicidal events was high for both ideation (intraclass correlation coefficient=0.9, p<0.001) and behavior (100% agreement). Not counted as suicidal events were instances of nonsuicidal self-injury, which was defined as engagement in self-injury with no intent to die, motivated by a desire to relieve negative affect or to obtain social reinforcement (
23,
24). Suicidal events were assessed by spontaneous report during the first half of the study and systematically at every visit during the second half of the study. Spontaneously reported events appeared to be representative of more serious suicidal adverse events (
19).
The sample was also characterized with respect to duration of depression and presence of DSM-IV comorbid disorders using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version, self-reported depression using the Beck Depression Inventory, suicidal ideation using the Suicidal Ideation Questionnaire–JR, and adolescent- and parent-reported family conflict using the Conflict Behavior Questionnaire, Adolescent Report and Parent Report versions (
25–
28). Self-ratings and interview ratings of symptoms and symptom severity were obtained at baseline, at 6 weeks, and at 12 weeks. In this sample, 83.2% completed treatment, and 96.1% and 96.8% were available for their 6-week and 12-week assessments, respectively.
Treatment
Participants were cross-tapered from their entry SSRI over the first 2 weeks and were seen by pharmacotherapists weekly for the first 4 weeks and every 2 weeks for the next 8 weeks. Those assigned to an SSRI (fluoxetine, paroxetine, or citalopram) received 10 mg/day for the first week and 20 mg/day for the next 5 weeks, and then, if the CGI-I score was 3 or greater, the dose was increased to 40 mg/day. Those assigned to venlafaxine had the following dosage schedule: 37.5 mg/day for 1 week and weekly increases of 37.5 mg/day until a dosage of 150 mg/day was reached at 4 weeks, with an option to increase to a dosage of 225 mg/day if the CGI-I score was 3 or greater. By 12 weeks, the mean dosage for SSRIs was 32.0 mg/day (SD=9.9), and for venlafaxine 201.6 mg/day (SD=35.0). Participants assigned to receive CBT had up to 12 sessions lasting 60–90 minutes, as described previously (
18). Participants in the pharmacogenetics study received an average of 9.6 CBT sessions (SD=2.5); there was no difference between medication cells. Adjunctive treatment (e.g., with trazodone or a benzodiazepine) was allowed for sleep and anxiety. In both the overall sample and in this subsample, the use of trazodone was associated with poorer antidepressant response and with self-harm (either nonsuicidal self-injury or suicidal) events (
18,
19).
Gene Selection
We targeted genes related to central serotonergic and noradrenergic neurotransmission, namely, tryptophan hydroxylase (TPH1, TPH2), the serotonin transporter (HTT, SLC6A4), serotonin receptors (HTR1A, HTR2A), the norepinephrine transporter (NET1, SLC6A2), the noradrenergic receptor (ADRB1), and monoamine metabolism (MAOA). We also studied genes involved in glutamatergic neurotransmission (GRIK2, GRIA3) and in the HPA axis (FKBP5) and genes that code for neurotrophic proteins (BDNF).
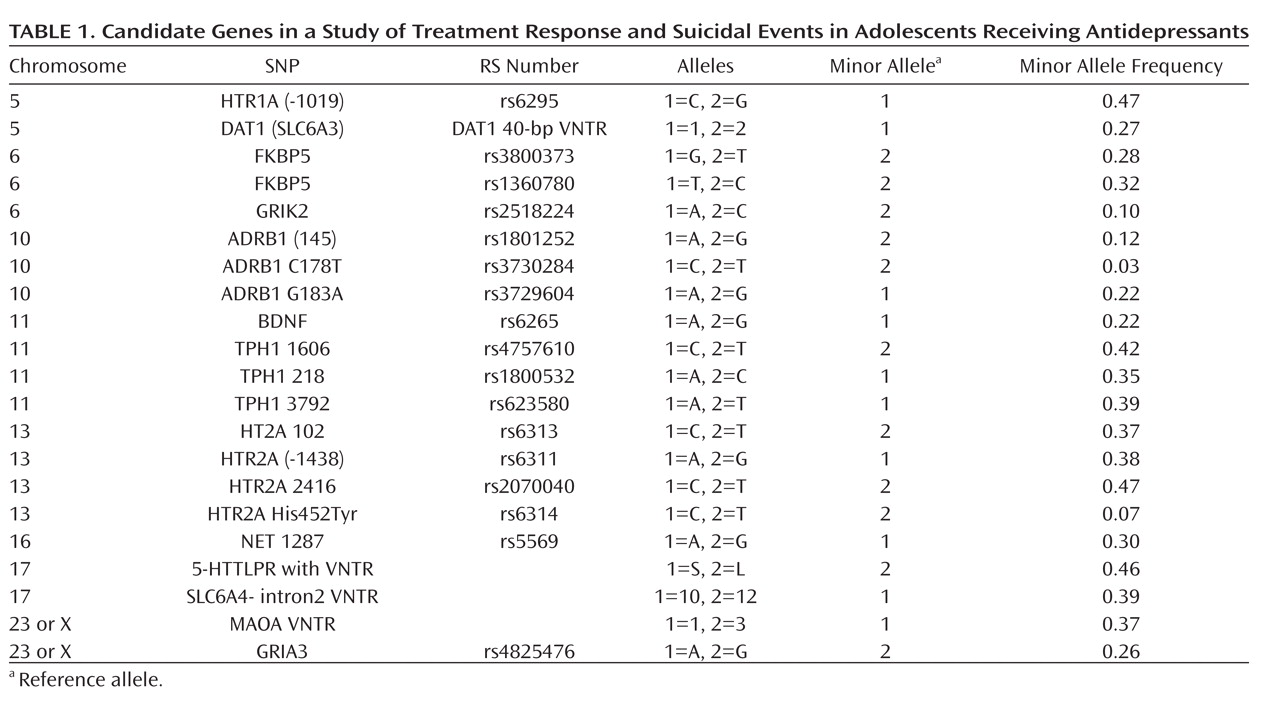
Table 1 lists the polymorphisms that we tested for each gene (rs) and the frequency of the minor allele.
DNA Samples
DNA was obtained from either blood or cheek swab from all participants. We collected 20 ml of whole blood in EDTA tubes, which were shipped to the Human Genetics Laboratory at the University of Pittsburgh for DNA extraction using the Puregene system (Gentra Systems, Minneapolis). Buccal cell DNA was extracted using a modification of this method. DNA was diluted to a standard 50 μg/ml, aliquoted, and frozen at –80°C until used.
Molecular Methods
All genotyping was carried out using standard polymerase chain reaction-based techniques that are routinely used in the Human Genetics Laboratory. DNA segments containing the target variable site were amplified using unique sequence flanking primers. Length polymorphisms (MAOA, DAT1 5′VNTR, HTT 5′VNTR) were genotyped by separation of amplimers on 3%–4% agarose gels and visualization of the ethidium bromide stained gel under ultraviolet light. All other sites are SNPs, which were genotyped by the fluorescence polarization method of Chen et al. (
29). Genotypes were assigned by direct comparison to sequence-verified control samples. Across genotypes, a median of 7% of samples could not be genotyped (range=0.0%–13.1%). A random 5% subsample of individuals was regenotyped with concordance observed for all duplicates. For none of the genotypes was genotyping success related to either of the two outcome variables for this study.
Statistical Analyses
Reported analyses were conducted on the 88.1% of the overall sample who were Caucasian (N=155), although all were repeated with the entire sample after controlling for ethnicity, which was assessed by participant self-report, with no difference in the results. The characteristics associated with treatment response and with the occurrence of suicidal events were assessed using standard univariate statistics. Hardy-Weinberg equilibrium was examined using exact tests (
30). For sex-linked polymorphisms, Hardy-Weinberg equilibrium was examined in females. SNPs with a minor allele frequency <0.05 were excluded (ADRB rs3730284T). All SNPs were in Hardy-Weinberg equilibrium. Single-SNP analyses were conducted using PLINK (
http://pngu.mgh.harvard.edu/~purcell/plink). For 80% power, an alpha of 0.001 to correct for the number of markers and outcomes, and a frequency of minor allele ranging from 20% to 40%, the power to detect an association between genotype and response was sufficient for a relative risk of 4 or higher, and the power to detect an association between genotype and suicidal events, given the lower frequency of the latter, was sufficient for a relative risk of 5.5 or higher.
Logistic regression analyses were used to examine the relationship between our outcome measures and the additive effects of each polymorphism, controlling for medication, treatment with CBT, and medication-by-CBT interaction and covariates that were significantly different on treatment response or suicidality (i.e., family conflict, trazodone). Because family conflict was associated with both response and suicidal events, we also controlled for family conflict-by-genotype interactions. For sex-linked polymorphisms, sex was included as a covariate in the models. We used permutation procedures (max[T] with 10,000 permutations) to generate significance levels empirically, which have the desirable properties of relaxing assumptions about Hardy-Weinberg equilibrium, dealing with rare alleles, and small sample sizes as well as providing a framework for correction for multiple testing. Two p values are computed: a pointwise estimate of the significance of each individual polymorphism and a p value that controls for the number of polymorphisms tested while preserving the correlation structure among them. We corrected the p value for examining two outcomes. This is conservative, since suicidal events were more common in those who did not respond to treatment (14/64 [21.9%] compared with 4/91 [4.4%]; χ2=11.2, df=1, p=0.001).
Results
Participant Characteristics
Patients who participated in the genetic study were similar to those who did not with regard to nearly all demographic and clinical variables and treatment assignment but were younger on average (15.7 years [SD=1.6] compared with 16.2 years [SD=1.5]; t=2.28, df=290, p=0.02) and were more likely to have at least one parent who graduated from college (48.8% versus 36.7%; χ2=3.94, df=1, p=0.047). Similar to the total sample, they were mostly female (70.5%), with moderate to severe chronic depression (CGI-S score, mean=4.4 [SD=0.6] and Children's Depression Rating Scale–Revised score, mean=58.8 [SD=9.8]) with a median duration of 2 years. A high proportion of participants entered the study with clinically significant suicidal ideation (59.1% had a score ≥31 on the Suicidal Ideation Questionnaire–JR) or a history of a previous suicide attempt (23.9%) or of nonsuicidal self-injury (38.7%). Anxiety disorders (31.7%) and attention deficit hyperactivity disorder (16.5%) were the two most common comorbid conditions. The 176 participants in the pharmacogenetics study were evenly distributed with respect to both CBT assignment (48.9%) and medication (50% to each medication condition), and the treatment groups did not differ with respect to baseline demographic or clinical variables.
Characteristics of those who responded. Participants who responded to treatment had a lower score for parent-child conflict on the Conflict Behavior Questionnaire–Adolescent Report (mean=6.7 [SD=5.9] compared with mean=10.1 [SD=6.2]; t=3.40, df=150, p=0.001), had a lower rate of previous nonsuicidal self-injury (29.2% versus 54.0%; χ2=9.46, df=1, p=0.002), and were less likely to have received trazodone (4.4% versus 25.0%; χ2=14.19, df=1, p<0.001). In the overall sample, CBT was associated with a greater response rate, which fell short of statistical significance in this subsample (54.9% versus 45.1%; χ2=3.08, df=1, p=0.08).
Characteristics of those with a suicidal event. A total of 18 participants experienced at least one suicidal event: four made suicide attempts, two communicated intent to attempt suicide, four had new-onset suicidal ideation (three with passive death wishes and one with ideation with a plan), and eight had worsening of their suicidal ideation by at least 2 points on the Brief Suicide Severity Rating Scale (four from passive death wish to ideation with a concrete plan, four from ideation to ideation with plan and intent). Those who experienced suicidal events were less likely to have a parent with a college education (23.5% versus 53.4%; χ2=5.38, df=1, p=0.02), had higher self-reported depression (Beck Depression Inventory score, mean=24.2 [SD=11.1] compared with mean=19.1 [SD=11.1]; t=–2.06, df=152, p=0.04), had higher suicidal ideation (Suicidal Ideation Questionnaire–JR score, mean=51.5 [SD=22.1] compared with mean=40.3 [SD=20.5]; t=–2.42, df=152, p=0.02), were more likely to have a history of nonsuicidal self-injury (77.8% compared with 34.3%; χ2=12.54, df=1, p<0.001), and had more child-reported parent-child conflict (Conflict Behavior Questionnaire–Adolescent Report score, mean=12.9 [SD=5.5] compared with mean=7.4 [SD=6.1]; t=3.61, df=150, p<0.001); those who had a suicidal event were more likely to have received trazodone (38.9% compared with 9.5%; Fisher's exact test, p<0.001).
Genetic Polymorphisms and Treatment Response
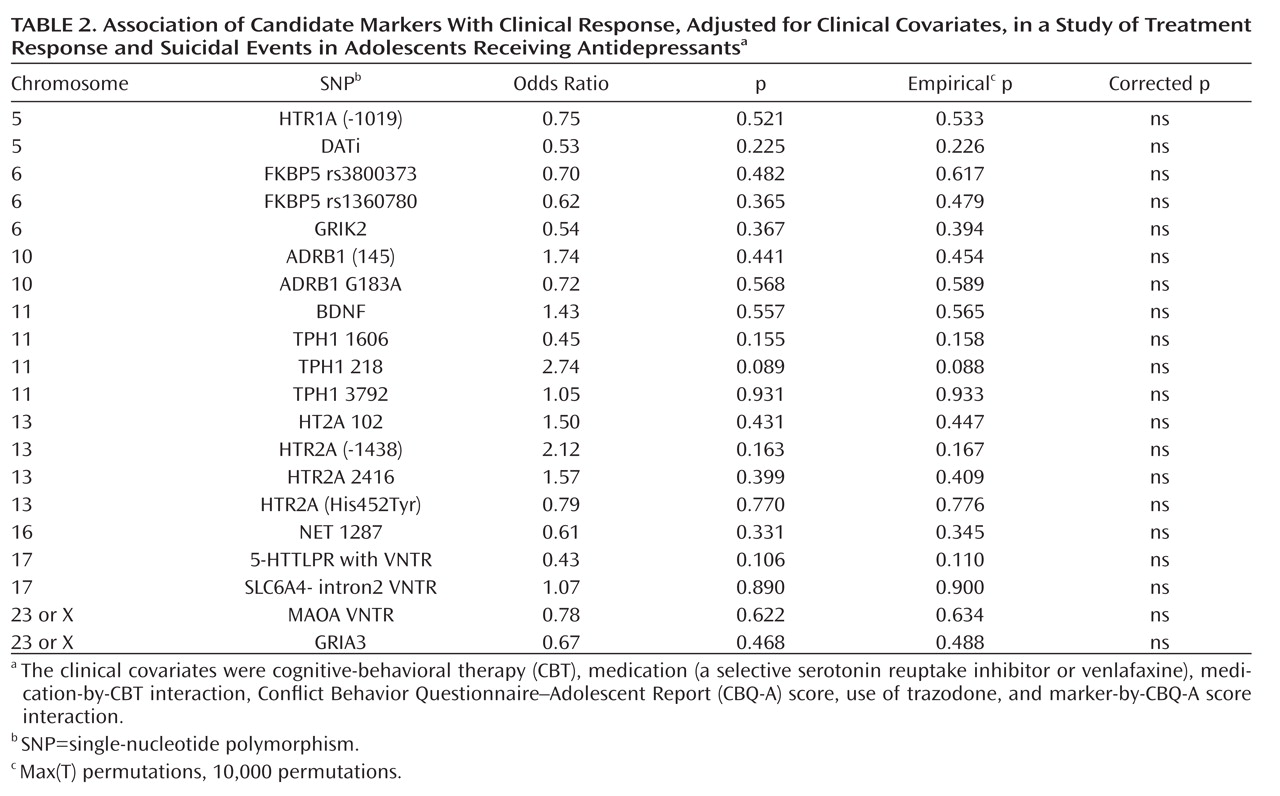
There were no univariate relationships between genotype and treatment response, although there was a nonsignificant trend for the FKBP5 rs1360780 and rs3800373 genotypes to be associated with a lower response rate. After controlling for CBT, medication, medication-by-CBT interaction, Conflict Behavior Questionnaire–Adolescent Report score, SNP-by-Conflict Behavior Questionnaire–Adolescent Report score interaction, and use of trazodone, there were no significant relationships between genotype and treatment response (
Table 2). There were no statistically significant gene-by-medication interactions with respect to genetic markers that we hypothesized would predict a differential response to venlafaxine (SNP-by-medication interactions for NET 1287 and ADRB SNPs).
Genetic Polymorphisms and Suicidal Events
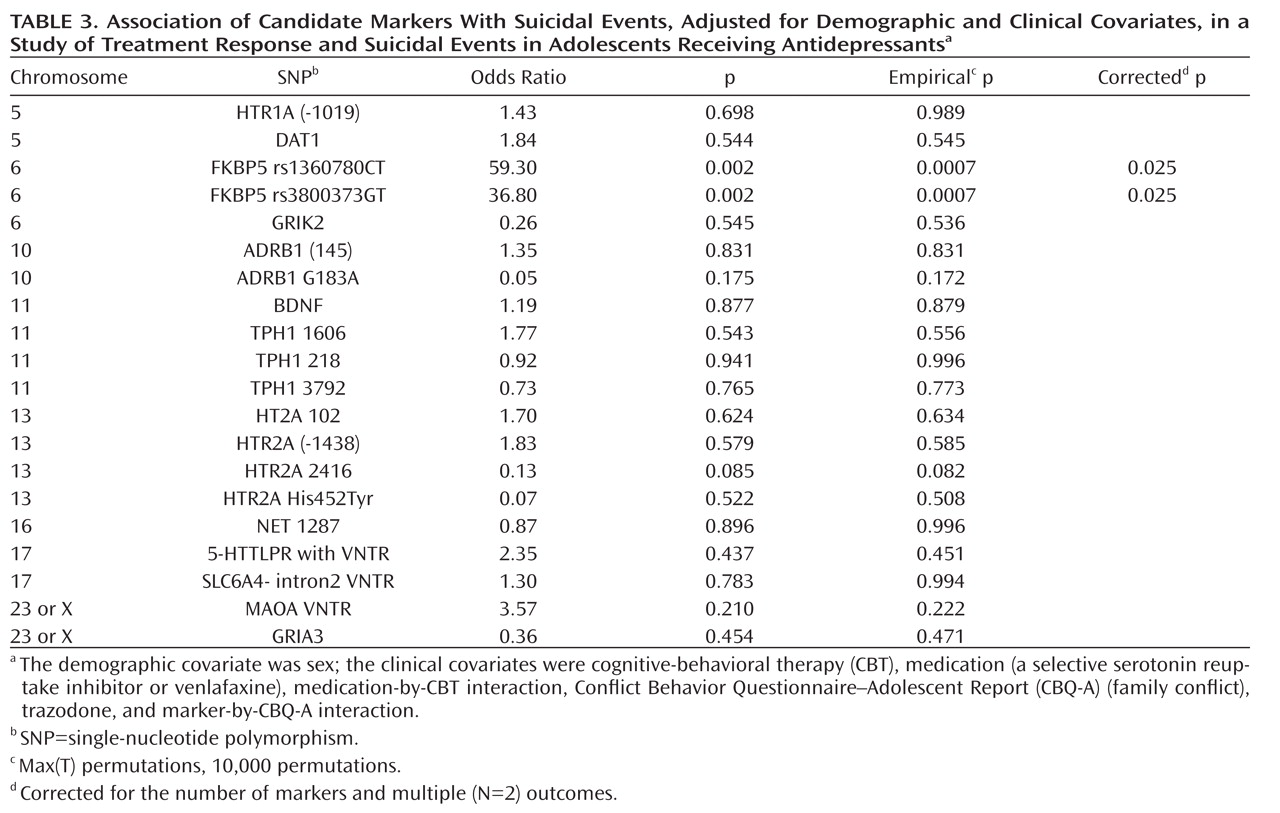
There was a significant association between the FKBP5 rs1360780 TT genotype and suicidal events (7/15 compared with 4/64 and 7/71, respectively, for the CT and CC genotypes; Fisher's exact test, p=0.001; TT versus CC/CT, Fisher's exact test, p<0.001), with similar findings for the FKBP5 rs3800373 GG genotype (7/14 compared with 4/61 and 7/78, respectively, for the GT and TT genotypes; Fisher's exact test, p<0.001; GG versus TT/GT, Fisher's exact test, p<0.001).These associations did not differ across treatment groups and were statistically significant even when restricted to participants who did not respond to treatment. The FKBP5 rs1360780 and rs3800373 genotypes showed similar relationships with suicidal events when these were assessed using spontaneous or systematic reporting. Genotype was unrelated to a previous history of attempts, nonsuicidal self-injury, and baseline suicidal ideation. With respect to the occurrence of suicidal events, there was an additive effect for both the FKBP5 rs1360780 (odds ratio=59.3, p=0.0007, corrected p=0.025, 95% confidence interval [CI]=4.7–743.6) and FKBP5 rs3800373 alleles (odds ratio=36.8, p=0.0007, corrected p=0.025, 95% CI=3.6–371.5), even after controlling for the effects of medication, CBT, CBT-by-medication interaction, use of trazodone, family conflict, and family conflict-by-genotype interaction (
Table 3). Follow-up analyses of the FKBP5 markers were consistent with a recessive effect for both the FKBP5 rs1360780TT (odds ratio=131.9, p=0.002, corrected p=0.004, 95% CI=6.4–2736) and for the FKBP5 rs3800373GG (odds ratio=79.1, p=0.003, corrected p=0.006, 95% CI=4.5–1389) genotypes. The addition of history of nonsuicidal self-injury as a covariate did not change these results. These two SNPs were in strong linkage disequilibrium (r=0.91).
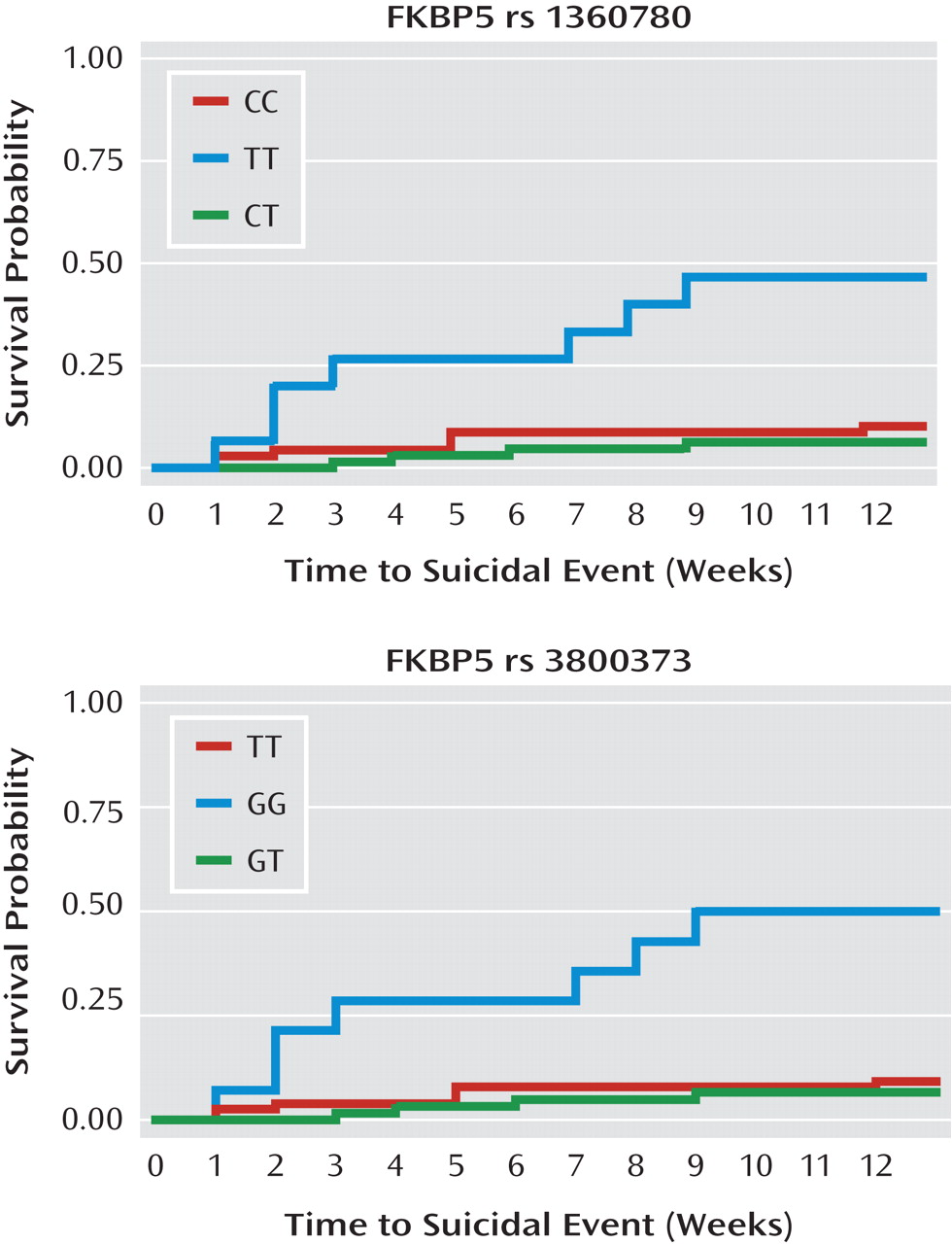
A higher hazard ratio and an earlier onset of a suicidal event was associated with the FKBP5 rs1360780TT and rs3800373GG genotypes (hazard ratio=9.30, 95% CI=2.7–31.8, and hazard ratio=9.70, 95% CI=2.8–33.24, respectively, p values <0.0001 [see
Figure 1]), and this effect persisted even after controlling for the effects of CBT, medication, medication-by-CBT interaction, trazodone use, Conflict Behavior Questionnaire–Adolescent Report score, and a Conflict Behavior Questionnaire–Adolescent Report score-by-genotype interaction (p values, 0.006 and 0.016, respectively).
Discussion
In this pharmacogenetic study of adolescent depression, we found an association between polymorphisms of the FKBP5 gene and the occurrence of suicidal events, most of them consistent with a recessive effect that persisted after controlling for treatment effects, and clinical covariates that were related to a suicidal outcome. In contrast, we did not find an association between any of the polymorphisms studied and clinical response. We discuss these findings in the context of the extant literature and the limitations and strengths of this study.
The limitations of this study make our findings necessarily preliminary. Our negative findings with regard to genotype and response could be due to the small sample size, which increases the likelihood of type II error. While the association we observed between the FKBP5 genotypes and suicidal events is statistically significant, it is based on a small number of suicidal events. Similar to many other pharmacogenetic studies, this study lacked a placebo condition, and it did not document drug exposure (
4–
17,
31). A placebo condition is necessary to determine whether suicidal events or response are truly related to treatment. Measurement of drug concentration is required to discriminate between true nonresponders and those who did not respond to treatment because of inadequate antidepressant exposure.
This study has several strengths. The youths in our sample are well-characterized and received carefully specified treatments. Our analyses, which controlled for the effects of treatment as well as other baseline differences in outcome that may affect treatment response and the occurrence of suicidal events, followed recommendations for pharmacogenetic studies (
31). Our definition of suicidal events was reliable, was in keeping with current nomenclature, and described events that were clinically significant (e.g., suicidal ideation with a plan and suicide attempts).
To our knowledge, the relationship between the FKBP5 gene and suicidal events has not been previously reported. These same markers, rs1360780T and rs3800373G, have been reported to be associated with the development of symptoms of posttraumatic stress disorder with current or past exposure to trauma in children and adults (
32,
33). Studies of adult populations have also found an association between these markers and a greater number of depressive episodes, more rapid response to antidepressants, and an elevated risk for bipolar disorder (
9,
12,
34,
35). Healthy adult subjects who are homozygous for each of these alleles (rs1360780TT, rs3800373GG) show a greater and more prolonged cortisol response during the Trier Social Stress Test compared with those who have complementary genotypes (
36). This is consistent with the function of the FKBP5 gene, which codes for a protein that decreases the sensitivity of the glucocorticoid receptor to the effect of corticosteroids (
37). In vitro exposure of cells to glucocorticoids induces an increase in FKBP5 mRNA, with FKBP5 rs1360780TT and rs3800373GG genotypes being the highest expressing variants. The same high expressing genotypes of FKBP5 that were associated with suicidal events in this sample have been shown to induce the greatest reduction in the sensitivity of the glucocorticoid receptor (
38). The association between suicidal events and a genotype associated with glucocorticoid receptor subsensitivity is consistent with previous studies linking suicidal behavior with insensitivity of the HPA axis to feedback and increased secretion of cortisol (
39).
This study suggests that further exploration of the relationship between genes in the HPA system, stress, and response to treatment for depression could be a fruitful line of investigation. It will be important also to replicate these results with an independent sample and to determine whether these findings are unique to the adolescent age group or are also found in older depressed patients.
Acknowledgments
The authors thank Beverly Sughrue for assistance in manuscript preparation, Nancy Petro for help in genotyping the samples, and the TORDIA families for their participation.