For more than 25 years the auditory paired-click paradigm has been used to study auditory processes in individuals with schizophrenia (
1). Subjects are presented with two auditory stimuli (S1 and S2) separated by 500 msec, and the amplitude of evoked brain activity 50 msec (P50) and 100 msec (N100) after each click is typically measured with EEG at electrode Cz. A higher than normal ratio of the S2 amplitude to the S1 amplitude is frequently observed in schizophrenia (
2). The functional implications of this ratio score deficit are currently in dispute. There is little evidence of a relationship between P50 ratio scores and positive or negative symptoms in schizophrenia (
3). A few studies have examined the association between paired-click ratio scores and cognitive measures, with some evidence of a relationship to processing speed (
4), attention (
5 and
6, although see
7), and explicit and implicit memory (
8).
There are at least two basic limitations to linking Cz activity and cognitive dysfunction. P50 recorded at the scalp is multidetermined, making it difficult to identify specific brain-performance relationships (
18), and several studies have shown that the P50 Cz ratio score is not a reliable measure in traditional psychometric terms (e.g.,
19–21, although see
22). Both limitations can be overcome by using source-localization methods. Studies using magnetoencephalography (MEG) to localize sources of brain activity during the paired-click paradigm in comparison subjects and schizophrenia patients focused on M50 and M100, the magnetic manifestations of the P50 and N100 neuroelectric components, and established that the bilateral superior temporal gyrus (STG) is the primary area active during the 50-msec period (
18,
23). Whereas group differences in the ratio score may be specific to the left STG at 50 msec, bilateral STG deficits exist at 100 msec (
24). In addition, source-localized M50 STG ratio scores are reliable (
21). In contrast to paired-click EEG studies, paired-click MEG studies have shown relationships with clinical measures: negative symptoms correlated positively with the right hemisphere M50 ratio score (
25).
Since our original study examining the relationship between EEG and MEG 50-msec ratio scores and cognitive abilities (
26), the size of our study group has increased approximately fourfold. In addition, the Wisconsin Card Sorting Test, which is sensitive to prefrontal/executive function (
27), was added to the cognitive battery. The present study examined the relationship between cognitive measures and EEG and MEG 50- and 100-msec ratio scores. Whereas the 50-msec component is thought to refiect bottom-up, preattentive processes (
28, although see
29–32), 100-msec activity may refiect the onset of top-down modulation by the frontal cortex (
33–35), a hypothesis supported by studies showing that N100 amplitude is influenced by attentional manipulations (e.g., references
33,
36,
37). Thus, 50- and 100-msec ratio scores may be differentially related to cognitive tests.
In the present study, MEG and EEG data were simultaneously collected during the paired-click paradigm, and Cz and MEG-derived STG activity was examined to uncover clinical relationships in a relatively large group of comparison subjects and patients. The following hypotheses were pursued: 1) in a replication of previous findings, patients with schizophrenia would show abnormally large P50, N100, left M50, and bilateral M100 ratio scores and 2) in keeping with 100-msec paired-click findings reported in previous studies (e.g., references
9,
38,
39), 100-msec ratio score group differences would refiect an encoding deficit. Given variable 50-msec amplitude findings, no predictions were made concerning group differences in the 50-msec S1 and S2 amplitudes (
3). Given that inadequate inhibition of redundant sensory information is thought to underlie attention dysfunction in patients with schizophrenia and that some studies have shown relationships between P50 and M50 ratio scores and attention, higher 50-msec and perhaps also higher 100-msec ratio scores and S2 amplitudes would be associated with impaired performance on attention measures (
4). As Cz ratio scores are unreliable, P50 and N100 ratio scores would be less (if at all) related to cognitive performance. In contrast, although EEG S1 and S2 amplitudes are determined by multiple sources, their reliability (
20,
21) means that these EEG amplitude measures might show associations with cognitive performance.
Method
Subjects
The participants were 79 patients with chronic schizophrenia (17 female) and 73 comparison subjects (20 female) (
Table 1). Recruitment procedures and information on inclusion and exclusion have been detailed previously (
24,
38); see the online data supplement for additional demographic information. In the patient group, 62 were treated with therapeutic doses of second-generation antipsychotics (clozapine, N=11; olanzapine, N=15; aripiprazole, N=8; risperidone, N=12; quetiapine, N=14; ziprasi-done, N=2), and 17 were receiving therapeutic doses of first-generation antipsychotics (fluphenazine, N=4; haloperidol, N=12; perphenazine, N=1). Fifty-four patients were diagnosed with the paranoid subtype, 23 with the undifferentiated subtype, and two with the disorganized subtype.
Paired-Click Paradigm
The procedure followed the protocol of Adler et al. (
40), in which 3-msec binaural clicks were presented in pairs (S1 and S2) with a 500-msec interstimulus interval and a variable intertrial interval of 7–11 seconds, averaging 9 seconds. The clicks were delivered through earphones placed in each ear canal. The peak intensity of the click was presented 30–40 dB above hearing threshold. If necessary, intensity was decreased to avoid elicitation of a startle refiex. Click pairs were presented until 150 trials were obtained without MEG or EEG artifact. The time to collect 150 paired-click trials did not differ between groups; for the comparison subjects the mean time was 32.43 minutes (SD=6.1), and for the patients it was 33.49 minutes (SD=6.8).
EEG Data Collection
EEG data were collected with Ag/AgCl electrodes. Impedances were below 10 kΩ. EEG was recorded by using two SynAmps amplifiers and SCAN software (Neuroscan, Herndon, Va.) with a bandpass filter (0.03–150 Hz) and a 60-Hz notch filter. The left mastoid and Cz were referenced to the right mastoid during recording and were re-referenced offiine to linked mastoids (
41). An electro-oculogram (EOG) (bipolar oblique: upper right and lower left sites) and electrocardiogram (ECG) (at the collarbone) were also obtained.
MEG Data Collection
MEG data were collected by means of a 122-channel biomagnetometer (NeuroMag, Helsinki) (A. Ahonen et al., unpublished article, 1992). EEG and MEG data were collected simultaneously with NeuroMag acquisition software and hardware (Elekta, Stockholm). After a bandpass filter (0.03–150 Hz) and a 60-Hz notch filter, EEG and MEG signals were digitized at 300, 467, or 500 Hz (data acquisition software and hard drive were upgraded during the course of data collection, and the digitization rate was increased). After the MEG session, structural magnetic resonance imaging (MRI) using a 1.5-T Picker Edge imager (Philips Health-care, Andover, Mass.) provided T
1-weighted, three-dimensional anatomic images. Details regarding online artifact rejection and other data collection procedures have been provided previously (
24).
Analyses of EEG Event-Related Potentials
Cz EEG P50 and N100 data were analyzed with custom MATLAB programs (MathWorks, Natick, Mass.). Individual S1 and S2 P50 and N100 averages were created by visually inspecting the raw data from each trial offiine and discarding trials with ocular or muscle artifacts or excessive alpha-band activity occurring from 300 msec preceding S1 to 300 msec after S2. The filter parameters for P50 were as follows: Fstop=3 Hz and Fpass=5 Hz for the high pass and Fpass=50 Hz and Fstop=60 Hz for the low pass. The filter parameters for N100 were as follows: Fstop=1 Hz and Fpass=2 Hz for the high pass and Fpass=38 Hz and Fstop=42 Hz for the low pass. Each filter was applied twice, once in the forward and once in the reverse direction, to increase roll-off and preserve latencies. Pre-stimulus baseline activity, computed as the mean amplitude from –100 to –10 msec, was removed.
The P50 response to S1 was defined as the most positive peak at electrode Cz occurring between 35 and 75 msec poststimulus. The P50 response to S2 was defined as the most positive peak that occurred within ±10 msec of the S1 peak. P50 S1 and S2 amplitudes were scored as the difference between the peak and the preceding negativity (N40) to ensure that the P30 component was not selected (
3). The N100 response to S1 was defined as the most negative trough at Cz occurring between 75 and 130 msec post-stimulus. The N100 response to S2 was defined as the most negative trough that occurred within ±10 msec of the S1 peak. N100 responses to clicks 1 and 2 were scored as the difference between the trough and the preceding positive peak (P50). Ratio scores were calculated for P50 and N100 by dividing the scores for S2 by the scores for S1.
Magnetic Source Analysis
To coregister the MEG and structural MRI data, three anatomical landmarks (nasion and right and left preauriculars) as well as more than 50 additional points on the scalp were digitized for each subject by using the Probe Position Identification System (Polhemus, Colchester, Vt.). The three fiducials were identified in the subject's MRI, and a transformation matrix that involved rotation and translation between the MEG and MRI coordinate systems was used.
A trial was rejected automatically if there was magnetic activity greater than 1,750 fT/cm in any MEG channel or if there was electrical activity between –110 and 110 µV peak to peak in the EOG channel. A –100 to –10-sec baseline adjustment was applied to the averaged MEG data. Prior to source localization, a 4–55 Hz bandpass filter was applied for M50 and a 2–40 Hz bandpass was applied for M100. In a few subjects, the high-pass filter setting was adjusted ±1 Hz to improve source localization.
The strength, location, and peak latency of M50 sources (35–75 msec poststimulus) and M100 sources (75–130 msec poststimulus) in the left and right hemispheres were determined by fitting a dipole separately over the left and right hemispheres and using subsets of 34 planar gradiometers over the temporal lobe. For modeling the S1 M50 and M100, 10 msec of data surrounding the M50 and M100 peaks were selected. Equivalent current dipoles were determined separately for each hemisphere. Only equivalent current dipoles with goodness of fit values (measures of the correlation between calculated and measured signals) exceeding 70% for S1 were accepted. The peak strength of the source, measured in nano-ampere-meters (nAm), over the 10-msec period was then determined. The S2 M50 and M100 measures were identified by using a procedure (
42) in which the location of the S2 dipole was assumed to be the same as that of the S1 dipole. To assure that the same component was chosen for both clicks, the S2 latency was required to be within ±10 msec for M50 and M100. In the event that no identifiable peak was available, the S2 amplitude was scored at the same latency as for S1. M50 and M100 ratio scores for each hemisphere and component were expressed as the S2 dipole peak source strength divided by the S1 dipole peak source strength.
Cognitive Measurements
To assess several cognitive domains, both groups were given the Wisconsin Card Sorting Test (
43), the WAIS-III digit span back (
44), Connors' version of the Continuous Performance Test (
45), the Trail Making Test A (
46), the Rey Auditory-Verbal Learning Test (
47), and the Wechsler Memory Scale—Revised (WMS-R) visual reproduction measure (
48). The Shipley Institute of Living Scale (
49) was administered to estimate IQ. An attention composite was derived from the time for the Trail Making Test A time (inverse) and the Continuous Performance Test hit rate and d′. A working memory composite was derived from Wisconsin Card Sorting Test perseverative errors (inverse) (
27) and digit span back total recall. Finally, a long-delay memory composite was computed from Rey's Auditory-Verbal Learning Test list A delayed recall and WMS-R visual reproduction delayed recall. The composite scores for attention, working memory, and long-delay memory were computed by z-scoring each test, adding the z-scores within each domain, and calculating the
z-score mean.
Statistical Analyses
Analyses of variance (ANOVAs) and t tests examined group effects on Cz and MEG amplitude and ratio score measures. To examine how cognitive ability may differ as a function of psychiatric status and ratio score, hierarchical regression was done in which ratio score was entered first, group second, and their interaction last, with each cognitive composite measure (attention, working memory, delayed memory) analyzed separately. For each cognitive measure, regressions were run separately for the two Cz ratio scores (P50 and N100) and four MEG ratio scores (left- and right-hemisphere M50 and M100). In addition, for each cognitive measure, separate regressions were run for S1 and S2 amplitude scores to assess whether relationships were specific to the ratio score or were instead better refiected by S1 or S2. As the primary goal was to identify associations between ratio scores and cognitive performance, ratio score was entered first in the regressions. The results, however, were essentially the same when group was entered first and ratio score second. See the online data supplement for information on outliers and EEG and MEG interrater reliability.
Discussion
As hypothesized, patients with schizophrenia had larger P50 Cz ratio scores. Although the group main effect for M50 ratio score was not significant, group variation in the difference between S1 and S2 amplitudes (S1 minus S2) for left but not right M50 was observed, supporting a left M50 paired-click deficit (hypothesis 1). Whereas the 50-msec S1 and S2 values reported in
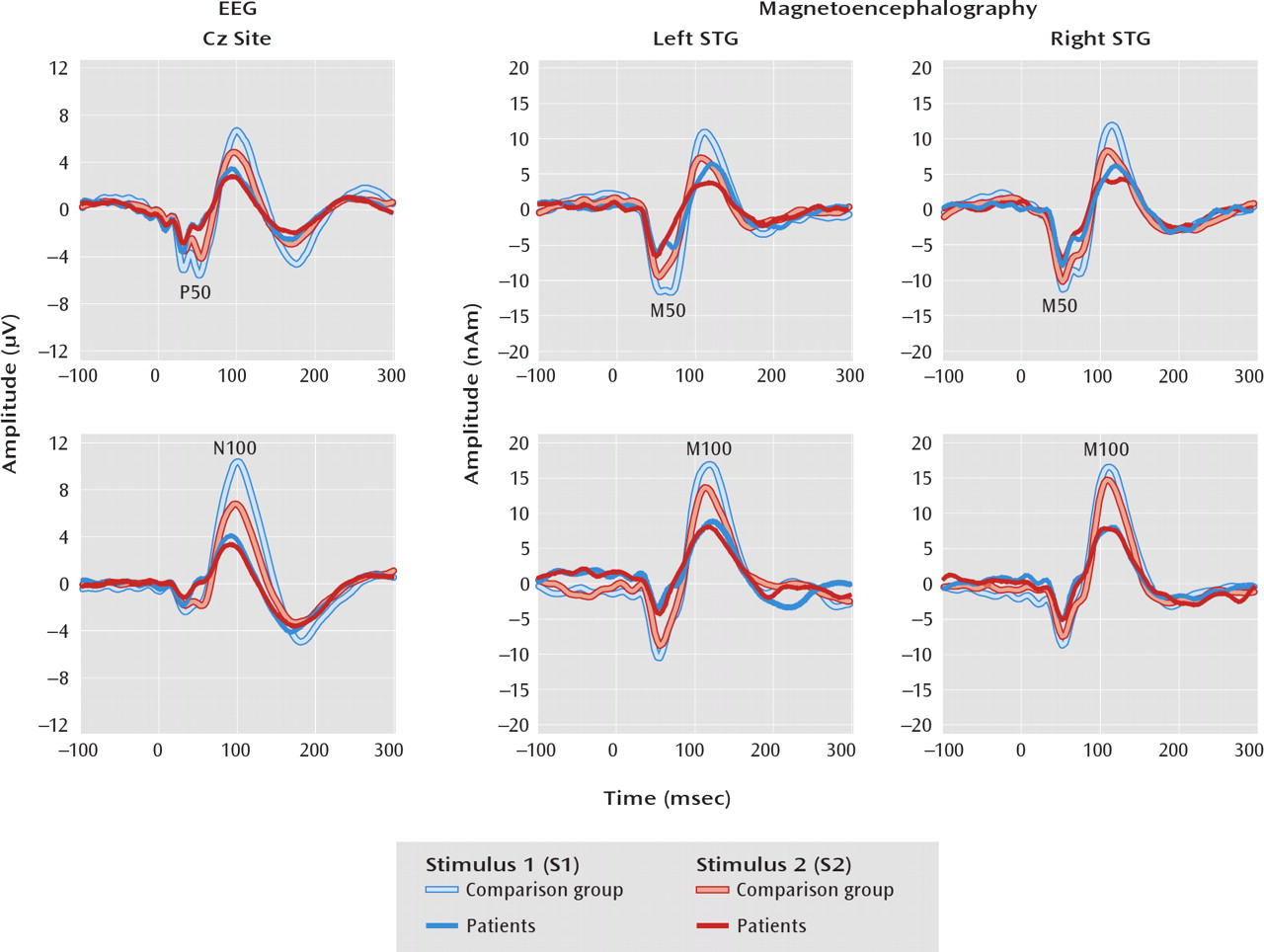
Table 2 as well as the
Figure 1 source waveforms suggest a left M50 S1 effect, analyses indicated that group differences in the ratio scores were not explained solely by either a pure encoding deficit (driven by S1) or a pure gating deficit (driven by S2). Therefore, the present findings do not resolve the 50-msec ratio score debate. The patients also showed larger N100 Cz ratio scores. For M100, group differences in the ratio score were observed bilaterally. As hypothesized, group differences in the 100-msec ratio score were due to a smaller S1 response in patients (hypothesis 2). Thus, ratio score group differences at 100 msec were explained by an encoding deficit.
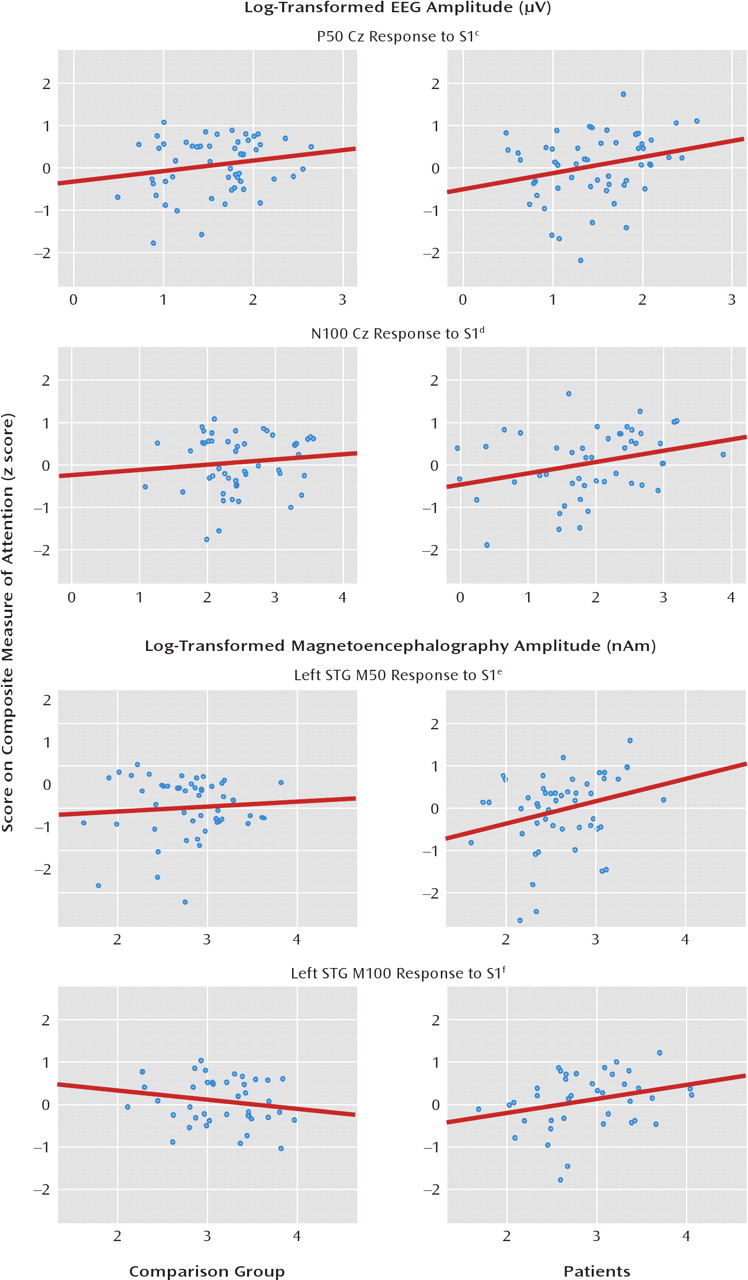
What is the functional significance of the paired-click findings? As 50-msec activity is thought to refiect sensory encoding, a relationship of the 50-msec and perhaps also 100-msec paired-click activity to attention was hypothesized, such that the less that redundant sensory information was inhibited (i.e., larger S2), the greater the attentional impairment (hypothesis 3). In the patients and comparison subjects, higher Cz P50 and N100 ratio scores were indeed associated with poorer performance on attention tests. For M50 and M100 STG measures, paired-click ratio scores were also associated with the attention composite, primarily in the patients. The second part of hypothesis 3 was not supported, as worse performance on attention tests was associated with a smaller S1 response rather than a greater S2 response (with the M50 relationship again observed only in patients). Thus, attention performance was predicted by the patients' ability to encode auditory information rather than their ability to filter redundant information. The lack of an association between STG S1 amplitude and attention in the comparison subjects may refiect the fact that most of the comparison subjects had normal STG activity. In any case, after the variance in ratio scores associated with general cognitive ability was removed, many of the associations of the 50- and 100-msec ratio scores with attention remained, suggesting that the association with attention was somewhat unique.
Ratio scores and amplitudes were also associated with working memory and verbal and visual long-delay memory. Only working memory added significant variance beyond that accounted for by general cognitive ability (IQ). Thus, the findings also reveal a nonspecific association of 50- and 100-msec electrophysiological measures (primarily S1) with general cognitive ability. It is interesting that associations were observed only with the ratio score or S1, again generally suggesting an association between encoding ability and cognitive performance.
Finally, hypothesis 4 received moderate support. First, to the extent that the Cz paired-click measure refiects brain activity only from primary and secondary auditory areas (especially true of 50-msec activity; see reference
23), present results indicate that the STG findings provide more information about paired-click group differences. For example, although a group difference in the P50 ratio score was observed, analyses of STG sources suggested that this was due to left and not right STG abnormality in the patients. In contrast, as group differences in the M100 STG ratio score were observed in both hemispheres, the Cz N100 findings more directly mirrored the STG findings. Second, whereas associations of the P50 and N100 Cz ratio scores and amplitudes with cognitive abilities were observed, the STG sources again provided more information. For example, although P50 and N100 S1 amplitudes were associated with attention (even after we removed IQ variance), STG analyses suggested S1 associations only in the left hemisphere and only in patients. To the extent that a detailed understanding of the specific brain areas associated with clinical measures is important, the present findings suggest the need to examine source rather than scalp activity.
Although in the present study MEG provided more specific information, the MEG and EEG findings were often similar. Thus, it is somewhat puzzling that so few studies have shown associations between paired-click activity and cognitive ability. There may be insufficient power in most EEG studies. The most extreme Cz example involves N100: although N100 was consistently associated with cognitive measures here, N100 ratio scores explained, at most, 5% of the variance in the cognitive scores. For a correlation of 0.22 (approximately 5% of the variance), a minimum of about 150 subjects would be needed to obtain a significant correlation (alpha=0.05, power=0.80), in line with the present subjects (comparison subjects plus patients) but far larger than groups in previous studies (see reference
3). The present findings suggest a larger effect size and thus a greater chance of observing associations when source activity is examined. For example, R
2 values of 15% for left M100 were consistently observed for attention and working memory measures, for which a group of 50 subjects would suffice (alpha=0.05, power=0.80). It should be noted that the present M50 results in the comparison subjects did not replicate our earlier finding (
26) in a much smaller but overlapping group of comparison subjects where worse M50 gating was associated with better working memory performance. The number of comparison subjects has increased fourfold since that study, and the present findings underscore the need to recruit relatively large subject groups to test relationships between paired-click measures and cognitive performance.
Although in the present study associations between auditory brain processes and cognitive ability were observed, the study design precludes causal claims. In addition, it is possible that a third, unstudied measure could account for electrophysiological and cognitive abnormalities. In particular, there is evidence to suggest that abnormal STG paired-click activity and impairment on cognitive tests in patients with schizophrenia may both be related to abnormal STG anatomy. In particular, low STG gray matter volume has been observed in many studies (for instance,
50–54). As 50- and 100-msec paired-click activity directly refiects gray matter activity, an examination of the relationship of 50- and 100-msec paired-click activity to STG structural measures is of interest. Available evidence also provides support for a relationship between STG gray matter structural abnormalities and functional impairment as assessed by psychophysiological measures as well as clinical measures. For example, lower than normal left temporal auditory P300 amplitude has been associated with smaller left posterior STG gray matter volume in chronic (
55) and first-episode (
56) schizophrenia. In addition, low left posterior STG gray matter volume has been associated with severity of thought disorder (
57), and in an MRI study the severity of thought disorder was negatively correlated with activation changes in left Brodmann's area 22 (
58). Thus, gray matter abnormalities may contribute to abnormal electrophysiology measures as well as to patient symptoms and cognitive abilities. In the present study, the observed left hemisphere M50 group differences may be due to structural STG hemisphere differences. Finally, it is worth noting that in the present study only S1 was associated with cognitive ability. To the extent that S1 activity primarily refiects local processes, whereas S2 may refiect local activity as well as inhibitory activity from the reticular formation and the thalamus (
59–61), hippocampus (
62,
63), or frontal cortex (
35), S1 and S2 processes would be expected to be differentially associated with cognitive measures.
As detailed in the introduction, a few other studies have demonstrated associations between Cz P50 and attention (
4,
5). In the present study a clear association between 50-and 100-msec ratio scores and performance on attention tests was observed, although primarily accounted for by S1 amplitude. To our knowledge, few studies have examined relationships between Cz N100 paired-click activity and cognitive measures (and we found no studies of associations with attention). Examining comparison subjects and patients with schizophrenia, Boutros et al. (
64) reported a relationship between N100 ratio scores and prefrontal cortex function (performance on the Wisconsin Card Sorting Test). In the present study, although no significant association was observed between performance on the Wisconsin Card Sorting Test and N100 ratio scores, an association between test performance and left and right STG M100 ratio scores was observed in the patients (see online data supplement). Another study examining only healthy subjects showed associations of N100 S1 amplitude and ratio score with a measure of working memory (
6), a relationship related more directly to stimulus processing properties (S1) than to N100 sensory gating. The present results generally replicated this finding, demonstrating an association between Cz N100 S1 amplitude and working memory performance.
In sum, the present findings indicate that the paired-click abnormalities predict cognitive deficits. In many instances, the cognitive impairments were more closely associated with encoding processes. The use of MEG source localization alongside scalp EEG provides larger effect sizes and more inferential specificity, which may explain why EEG-only studies have been inconclusive regarding an association between paired-click activity and clinical measures.