While both greater maternal age and paternal age have been found to increase the risk of schizophrenia, the effect of maternal age has been found largely to be explained by the effect of paternal age (
1,
2). “Effect of paternal age” will in the following refer to the association between increasing paternal age and increased risk of schizophrenia. In 2001 Malaspina and colleagues reported the association between paternal age and schizophrenia and hypothesized that de novo mutations in paternal germ cells, which accompany increasing paternal age, are responsible for the association (
3). This hypothesis seems in accordance with the fact that the rates of schizophrenia are similar across populations and stable over time, although schizophrenia leads to lower fertility (
2). There is, however, no direct evidence that the effect of paternal age is due to de novo mutations. Although it has been suggested that de novo mutations are too rare to have any significant effect on the maintenance of schizophrenia (
4,
5), McClellan et al. argued for a “common disease—rare alleles” model for schizophrenia (
6). According to this model, rare alleles with large effects are involved in the etiology of a substantial proportion of schizophrenia cases, and de novo mutations contribute to the persistence of rare alleles with large effects and, hence, to the persistence of schizophrenia. The association between greater paternal age and greater schizophrenia risk is seen as indirect evidence in favor of this model. However, this argument again rests on the unconfirmed assumption that the association is
caused by de novo mutations, and therefore evaluating this assumption can have implications for theories and research strategies regarding the genetic causes of schizophrenia.
In 1966, a study of persons with schizophrenia and their nonschizophrenic siblings found no difference in the paternal ages for the two groups (
7). The tabulations in the article did not point toward paternal age as a cause of whether one or another sibling of the same father becomes diagnosed with schizophrenia; rather, the author argued, the association between paternal age and schizophrenia risk could be caused by the influence of a predisposition to schizophrenia on the parents' personality, giving them a tendency to postpone childbearing. On the other hand, it has been argued that this hypothesis would lead to a stronger paternal age effect among familial cases, in contrast to sporadic cases (
8).
The rate of de novo mutations increases with increasing paternal age at conception. In contrast, the selection into late fatherhood, as measured by the age of the father when he had his first child, is constant for all the children of the same father, regardless of the father's actual age at conception. An independently increasing effect of paternal age at birth of the proband would favor the de novo mutation hypothesis, whereas an independently increasing effect of the age at which the father had his first child would favor the hypothesis of selection into late fatherhood.
Method
Study Population
Using data from the Danish Civil Registration System (
14), we identified all persons born in Denmark from Jan. 1, 1955, to Dec. 31, 1992, and alive at their 15th birthday. So that links to their parents would be as complete as possible, we restricted the study sample to individuals with Danish-born parents. The register includes personal identification number, gender, date of birth, continuously updated information on vital status, and parents' identification numbers. The identification number is used as a personal identifier in all national registers, enabling unique linkage between registers.
Assessment of Schizophrenia and Mental Illness
The members of the study population and their parents and full and half-siblings were linked with the Danish Psychiatric Central Register (
15), which contains data on all admissions to Danish psychiatric inpatient facilities since April 1, 1969. From 1995 on, information on outpatient visits to psychiatric departments was included in the register. From April 1969 to December 1993, the diagnostic system used was ICD-8, and from January 1994, ICD-10 was used. Cohort members were classified as having schizophrenia if they had a record of psychiatric in- or outpatient care for the disorder (ICD-8 code 295 or ICD-10 code F20). The date of onset was defined as the first day of the first contact (in- or outpatient) with a diagnosis of schizophrenia. Parents and siblings were categorized hierarchically with a history of schizophrenia (ICD-8 code 295 or ICD-10 code F20), schizophrenia-like psychosis (ICD-8 code 297, 298.39, or 301.83 or ICD-10 codes F21–F29), or other mental disorder (any ICD-8 or ICD-10 diagnosis) if they had been admitted to a psychiatric hospital or had received outpatient care for one of these diagnoses.
Study Design
A total of 2,245,463 people were followed up from their 15th birthday until the first diagnosis with schizophrenia, death, emigration from Denmark, or Dec. 31, 2007, whichever came first.
Among the 1,163,622 children who had an older sibling with the same father, the paternal age and paternal age at first child could be evaluated for separate independent effects.
Statistical Analyses
The incidence rate ratios for schizophrenia were estimated by using Cox regression in Stata 10 (Stata, College Station, Tex.). The basic model was adjusted for sex by means of separate underlying hazard functions, for age in the nonparametric part of the Cox model, and for calendar time as a time-dependent variable. Calendar time was categorized in 2-year groups during the 1990s to account for changes in the ICD system and in 10-year groups in other periods. Paternal age was categorized as less than 20, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, and 55 or older, and maternal age was categorized as less than 20, 20–24, 25–29, 30–34, 35–39, and 40 or older. As reference ages, we used the most common age categories, which for both paternal and maternal ages was 25–29 years. Familial psychiatric history was coded as a time-dependent variable for mothers, fathers, and siblings separately. Degree of urbanization at the place of birth was represented by five categories: capital city, capital suburb, provincial city with more than 100,000 inhabitants, provincial town with more than 10,000 inhabitants, and rural area.
Children of the same father comprised a cluster, and allowance for a possible within-cluster dependence was made by using robust standard error estimates provided by the cluster option in Stata. As a sensitivity analysis we further restricted the samples to children of the same father who were full siblings, which did not change the presented results. The proportional hazards assumptions were tested according to Schoenfeld's residuals (
16). The psychiatric history of fathers and mothers did violate the assumption, i.e., these effects varied with the age of the child. Supplementary analyses were performed to allow these variables to have a nonproportional hazard by stratifying the underlying hazard, which did not change the presented results. Power calculations were performed according to the method by Hsieh and Lavori (
17), which is implemented in the Stata procedure stpower cox.
Results
In this population-based cohort of 2.2 million persons born in Denmark between 1955 and 1992, a total of 14,211 persons developed schizophrenia during the 43.7 million person-years of follow-up from 1970 to 2007.
Table 1 shows characteristics of the study population according to the age of the father.
Table 2 shows the incidence rate ratios according to paternal and maternal age groups, with children born to parents between 25 and 29 years old as the reference groups. Adjusted for sex, age, and calendar time (first adjustment), the incidence rate ratio was lowest for paternal and maternal ages between 25 and 29 years, after which the incidence rate ratio increased with increasing paternal age. A less clear and smaller increase with increasing maternal age was observed. The offspring of mothers or fathers younger than 24 years were also at significantly higher risk of schizophrenia. When the parental ages were adjusted for each other (second adjustment), the increased risk associated with greater maternal age vanished, while greater paternal age still increased the risk of schizophrenia. The further adjustments for urbanicity and family history of psychiatric illness reduced the effects (final adjustment). The additional adjustment for family history, not only for schizophrenia but for mental illness in general, did not change the estimates of paternal age notably (results not shown).
The effect of paternal age did not differ significantly by proband sex (p=0.73) or by family history of schizophrenia (p=0.38).
When the effect of paternal age was allowed to differ according to maternal age, with all the confounders previously mentioned accounted for, the interaction between paternal and maternal ages was statistically significant (p=0.005).
Paternal Age at Proband's Birth and at Birth of Father's First Child
Among the 1.2 million second- or later-born children (a restriction necessary in analyzing the effect of both the father's age at the proband's birth and the father's age when he had his first child) who were born in Denmark between 1955 and 1992, 7,156 developed schizophrenia during the 21.9 million person-years of follow-up in 1970–2007.
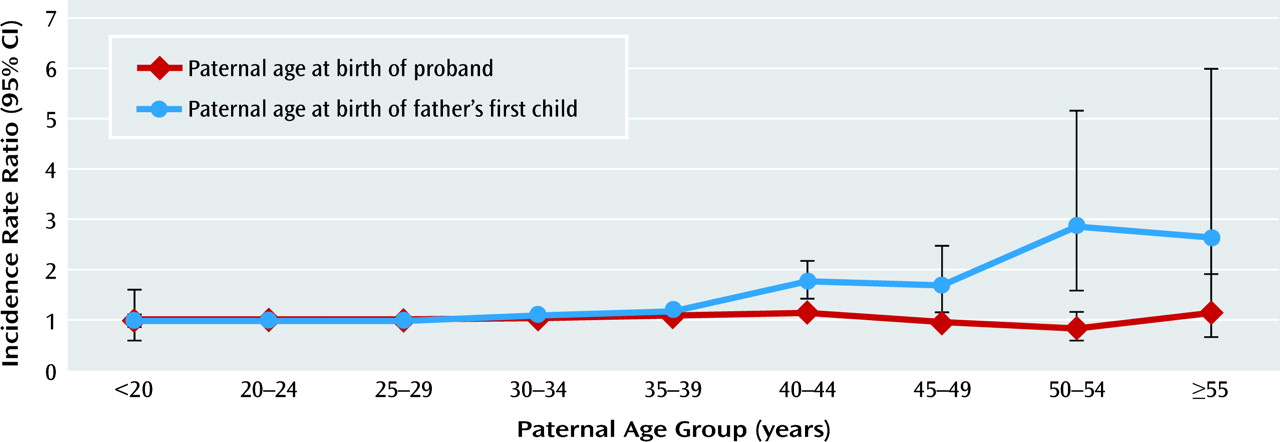
Table 3 shows the characteristics according to paternal age and paternal age when the father had his first child. The crude incidence rate of schizophrenia shows greater variation with the father's age at the time of his first child's birth than with his age at the time of the proband's birth, thus suggesting that the former is more important in terms of the proband's risk of schizophrenia.
To assess the generalizability of results from this subcohort of second- or later-born children, we estimated the fully adjusted effect of paternal age, which shows a pattern similar to that in the total cohort (see model 1 in
Table 4). In this population we distinguished the effect of paternal age at the time of the proband's birth from the potential effect of the age at which the father had his first child.
We calculated the power to detect an effect of paternal age of the size found in the full cohort (
Table 2) if the alternative is the truth, i.e., that the paternal age effect is preserved when the analysis is restricted to second- or later-born children and adjusted for the correlated variable paternal age at which the father had his first child. Calculations were conditional on the observed failure probability, the observed distribution in the paternal age categories, and a two-sided significance level of 5%. In the four groups of paternal ages between 30 and 50 years, the power to find a true paternal age effect was 100%, and even in the extreme category with paternal age above 55 years, power exceeded 90%.
Model 2 in
Table 4 shows the mutually adjusted incidence rate ratios for paternal age and paternal age at which the father had his first child; the analysis was adjusted for all the confounders mentioned and, in addition, for maternal age at the birth of the mother's first child. The risk of schizophrenia did not depend on paternal age at the birth of the proband. In contrast, the proband's risk of schizophrenia increased significantly with increasing paternal age at which the father had his first child (
Figure 1). Unlike the results in
Table 2, these findings indicate that none of the confounders substantially changed the effects of paternal age or paternal age when the father had his first child. In an analysis adjusting for sex, age, and calendar time only, the effect of paternal age at the birth of the oldest paternal sibling was as statistically significant as, but slightly less steep than, the adjusted one presented in
Table 4. The effect of paternal age was not modified significantly by paternal age at first child or vice versa (p=0.18). In stratified analyses, for a given paternal age at the birth of the oldest paternal -sibling (below 20, 20–29, 30–39, 40–49, and 50–59) there was no effect of paternal age (results not shown).
To assess residual confounding from familial history of psychiatric admissions caused by admissions before the start of the register, we restricted the cohort of probands to those born between 1970 and 1992. The results were unchanged, which suggests that the lack of register information on family members before 1970 had not influenced the results.
Overall, the incidence rate ratio increased 1.22 fold (95% confidence interval, 1.15–1.29) per 10-year increase in paternal age at the time of the first child's birth (
Table 5). The effect of paternal age at which the father had his first child differed significantly by sex (p=0.02) but not by family history of schizophrenia (p=0.65) or age at onset (p=0.51), according to Cox regression analysis of effect modification.
Discussion
In accordance with previous studies, our investigation confirms that greater paternal age is associated with a higher risk of schizophrenia. However, when the independent effects of paternal age at the proband's birth and age at which the father had his first child were assessed, there was no association with age at birth of the proband, whereas there was a strong association with the age at which the father had his first child. Not only did age at first child have an independent effect, but paternal age had no independent effect in subgroups of age at which the father had his first child. This is the opposite of what would be predicted if de novo mutations in paternal germ cells were a major reason for the effect of paternal age. Conversely, our findings are compatible with the hypothesis that late paternity is associated with a predisposition to schizophrenia.
As found in earlier studies, family history of schizophrenia was a confounding factor for paternal age. However, we found that family history of mental disorders in general did not further confound the effect of paternal age. Still, there could be residual confounding, as we were able to adjust only for psychiatric history leading to psychiatric treatment.
It is important to note that in the initial analyses, similar to those in previous studies, our results were in agreement with earlier findings (
18). This reassures us that our other findings plausibly would apply to other studies of paternal age and schizophrenia.
As in earlier studies, there was no statistically significant difference in the effect of paternal age between men and women (
3,
11,
12). Like Byrne et al. (
11), we found the same effect of paternal age in persons with and without a family history of schizophrenia; in contrast, a Swedish register-based study with less power than ours found no effect of paternal age in persons with a family history of schizophrenia (
12).
The effect of paternal age did depend on the maternal age, with strong statistical significance. A cross-tabulation of paternal versus maternal age by Malaspina et al. (
3) did not reveal such a dependence.
When the analyses were restricted to the second- or later-born children (presented in
Table 3), the number of cases, as well as the amount of risk time, was reduced by half. Still, this leaves us with 10 times as many cases as in the largest population-based cohort study of schizophrenia reported in the literature so far, and to find a preserved effect of paternal age in a model including the correlated variable paternal age at first child, the power exceeded 90%. In this model, surprisingly, the adjustments attenuated the effect of paternal age at birth of the proband only, whereas for paternal age at first child the estimates were unchanged by the adjustments. Another important issue could be the correlation between paternal age at the proband's birth and paternal age at the first child's birth, which has an effect on power. In this setting, the impact on the number of cases required to preserve a given power, when we adjust for paternal age at which the father had his first child, is fivefold (
17), a factor that is accounted for in the power calculations. Accordingly, in this large population-based study we had power to estimate the correlated variables, and we found there was no effect of paternal age within any subcategory of paternal age at the father's first child. The effects of paternal ages could be distorted by an effect of short birth interval (
19); however, an additional analysis adjusting for preceding birth interval did not explain the effect of paternal age at the birth of the first child. There was a tendency toward increasing paternal age with calendar time, but a more or less detailed adjustment for calendar time did not influence the estimated effects of paternal age.
If we assume that the age at which the father had his first child captures all of the effect of paternal age and increases the risk of schizophrenia equally per 10-year increase in age, the increased risk was higher in women than in men. The risk also increased among people with a familial history of schizophrenia, which indicates that paternal age is not a proxy for unmeasured family history. Paternal age at first child had a nonsignificantly stronger effect on early-onset schizophrenia than on onset after age 20 years; this resembles results found in bipolar disorder (
20).
It has been suggested that epigenetic processes, such as imprinting errors, contribute to the effect of paternal age on schizophrenia (
21). However, as in de novo mutations, the mechanism in this process would cause an increasing error rate with increasing paternal age at conception. Consequently, this hypothesis leads to the same prediction as the hypothesis of de novo mutations, and therefore our findings also argue against this hypothesis as an explanation of the association between paternal age and the risk of schizophrenia. Of course, this does not exclude the possibility that epigenetic changes as well as de novo mutations per se may contribute to schizophrenia risk, but our findings make it unlikely that they explain the association with paternal age. Even after family history of psychiatric illness in general was taken into account, the effect of paternal age at the father's first child remained statistically significant. Genetic stratification cannot be excluded as part of an explanation; one may hypothesize that a proportion of Danish men carry clinically unexpressed genotypes that are associated with schizophrenia as well as with reproduction at a later age. Also, the association could be mediated through other pathways; for example, factors leading to reduced male fertility, e.g., environmental pollutants or infection, could also be associated with increased schizophrenia risk in the offspring, or personality traits causing difficulties in finding a partner could be associated with higher genetic risk for schizophrenia.
Apart from Granville-Grossman (
7), we know of no one who has used siblings of the same father to distinguish whether an effect of paternal age depends on the age at conception or the age at which the father had his first child. Our study therefore needs replication.
In conclusion, in line with other studies, our results show an association between paternal age and schizophrenia. However, the age of the father when he had his first child ever, rather than his age at the birth of the subject under study, explained the association between paternal age and the risk of schizophrenia. In the absence of other evidence linking de novo mutations to schizophrenia, we believe there is a need to search for other risk factors for schizophrenia, factors that are associated with delayed fatherhood, in order to explain the effect of paternal age on schizophrenia risk.