Hallucinations can be defined as perceptions without corresponding sources in the external world. This feature represents one of the main positive symptoms of schizophrenia spectrum disorders, and 60%–70% of patients meeting the diagnostic criteria for this pathology experience hallucinations (
1). Even though they may involve any of the senses, auditory verbal hallucinations (AVHs) are most prevalent in such a psychiatric context. Patients experiencing AVHs usually describe hearing words, sentences, and conversations that are often intrusive or comment on their thoughts. In about 25% of patients, this symptom can be drug-resistant and become chronic (
2), causing an impaired quality of life. The pathophysiology of AVHs is still poorly understood, even though neuroimaging exploration has expanded in the recent decades to address various morphometric, functional, and connectivity issues in patients with schizophrenia. Many underlying mechanisms for AVHs have been proposed (
3), most of which are not mutually exclusive. Three main mechanisms are briefly presented in the present meta-analysis. First, some authors have postulated that AVHs could result from aberrant perceptions generated in auditory regions. Primary support for this theory came from the generation of involuntary auditory or verbal material during per-operative electrical stimulations of the temporal cortex in nonschizophrenia subjects (
4). Another influential hypothesis concerning the origin of AVHs is external misattribution of self-inner speech. According to this model, patients with schizophrenia are unable to identify their own thoughts as self-generated and, furthermore, interpret them as intrusive alien voices within their heads (
5). Finally, possible dysfunctions in the neural substrates of episodic verbal memory have been proposed to account for the involuntary emergence of AVHs (
6).
An initial reappraisal of the functional imaging procedures developed to test these pathophysiological hypotheses allowed us to conceptually distinguish between two main study categories. First are the cognitive studies comparing hallucinators and nonhallucinators. These studies, called trait-studies, investigate the neural bases of the susceptibility to hallucinate, independent of the subjects' experience during scanning. Second are state-studies conducted during the occurrence of an AVH, which are of particular significance for our purpose, since they directly measure brain activations associated with symptom emergence. However, the problem of disentangling the previously evoked hypotheses regarding the origin of AVHs is actually exacerbated by the fact that only a few studies have directly explored the AVH state, resulting in inconsistencies between findings. Some authors have identified restrictive activations in the Heschl's gyrus (
7,
8) or in Broca's area (
9,
10) in support of a strict sensory or motor origin for AVHs. Meanwhile, other studies have identified more distributed fronto-temporal networks coupled with subcortical structures (
11–13). Notable reasons for this difference include the fact that an AVH constitutes an unpredictable subjective event, for which various detection designs have been proposed. In a first subset of studies, symptom occurrence was signaled by pressing a response button when experiencing an AVH during scanning (
7,
10,
13,
14). Other authors have employed a discontinuous acquisition method: the random sampling approach, in which a large number of functional magnetic resonance imaging (fMRI) volumes were acquired at random intervals during AVHs (
11,
15). Patients reported their sensory experiences immediately after each acquisition. Besides these hypothesis-driven methods, some studies have used more data-driven methods, such as spatial Independent Component Analysis. This method does not rely on a predefined model of brain activity (
16). In these studies, AVH occurrences were sometimes signaled by button presses (
8,
17) or on the basis of a posteriori standardized interviews (
12,
18) to help select the components of interest.
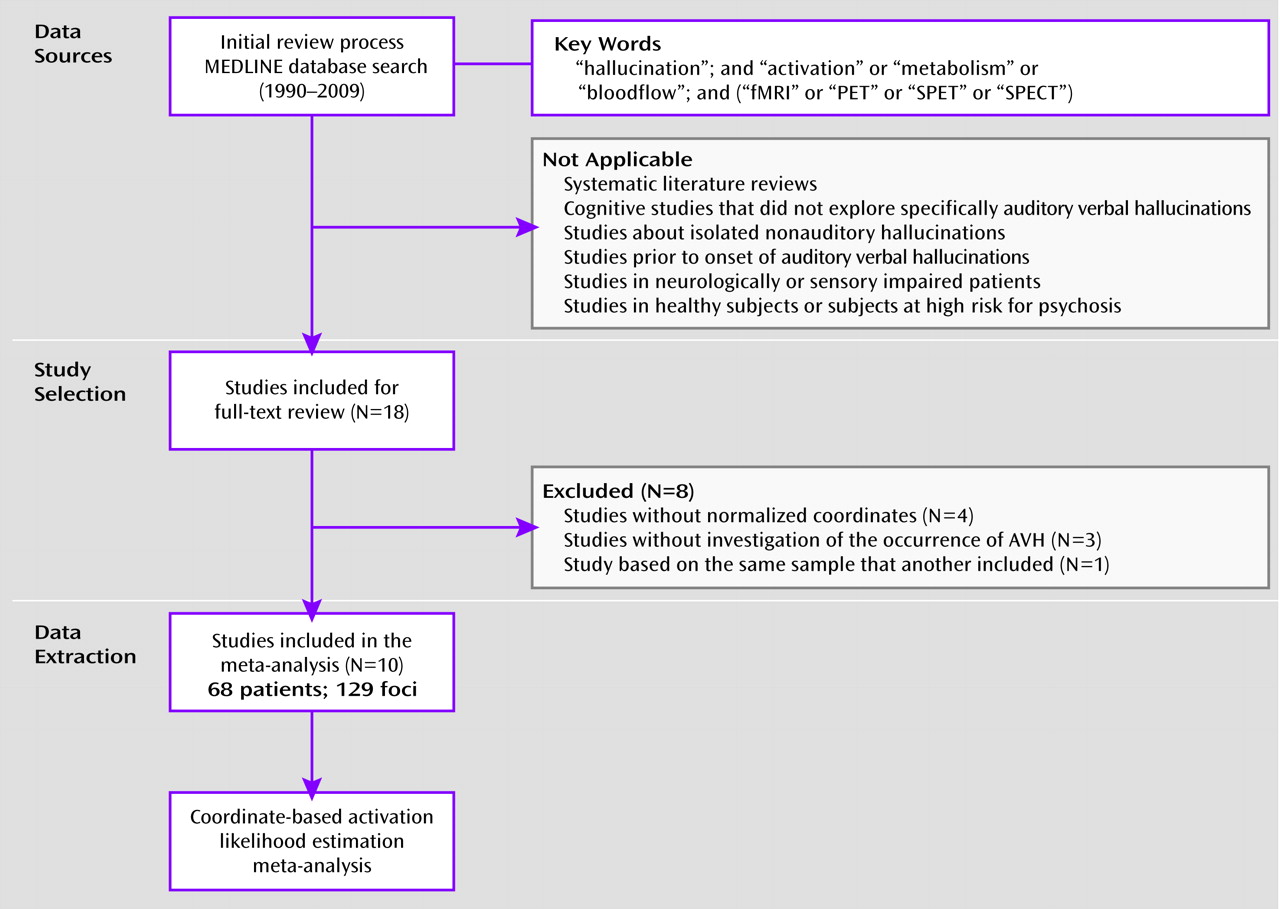
Another plausible explanation for disparities across studies could be considerable interindividual variability of the brain areas involved in AVHs, supporting several possible underlying mechanisms. However, since the available reports often included small samples or did not perform group analyses, it remains difficult to draw definitive conclusions and generalize to the whole population of patients based on the findings. Quantitative meta-analytic techniques have been precisely developed to provide objective measures of functional data and resolve such conflicting views. In the present review, we employed a revised version of the activation likelihood estimation algorithm (
19) to describe the brain locations most consistently active during the activation likelihood estimation state across studies. Activation likelihood estimation was recently judged to be the best coordinate-based meta-analysis method when compared with the gold standard of image-based procedures (
20). This method allowed us to perform a random-effects meta-analysis and to control for one of the major drawbacks of the previous fixed-effects procedures (i.e., their strong tendency to be dominated by one or a few individual studies) (
21). In the present meta-analysis, we postulated that AVHs in patients suffering from schizophrenia spectrum disorders could rely on the interaction between several brain areas involved in a widespread cortical network rather than on restricted sensory or motor activations.
Discussion
In the present review, we used coordinate-based meta-analysis to determine the brain areas predominantly recruited during AVHs in people suffering from schizophrenia spectrum disorders. Critically, the random-effects activation likelihood estimation method used allowed generalization of the results to the entire population of studies from which the analyzed experiments were drawn. Its robustness was reinforced by a weighting procedure of the localizing power in favor of the studies with larger sample sizes.
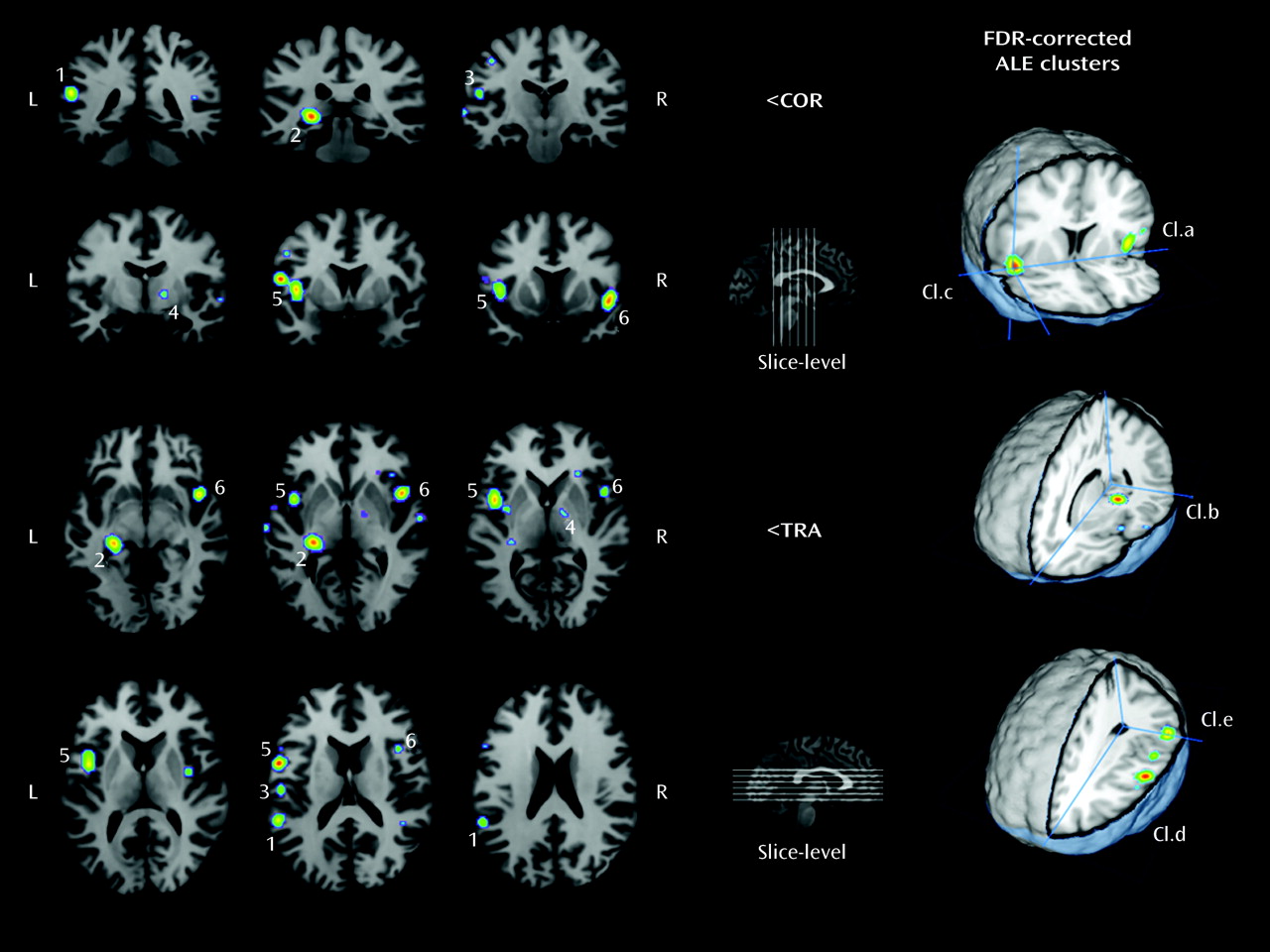
This meta-analysis first identified a widespread set of dysfunctional language-related areas that present increased activity when patients experience AVHs. The largest clusters were identified in cortical areas involved in speech generation. The left pars opercularis (Brodmann's area 44), located in the inferior frontal gyrus, is part of Broca's area. This region is bounded by the premotor precentral gyrus (Brodmann's area 6) to its posterior and in depth by the anterior insula (Brodmann's area 13). Overall, the insula constitutes one element of the homologous region of Broca's area on the right side of the brain. Lesion and functional imaging studies have revealed the critical involvement of this extended Broca's convolution in syntactic processing (
75–77) and also during verbal imagery (
78). In up to 90% of healthy right-handed subjects, this region shows strong functional left lateralization (
79). Some authors have suggested that reduced language lateralization in the frontal lobes of schizophrenia patients could account for the emergence of AVHs (
80–82). Our meta-analytic data, which show significant activations during AVHs within the left Brod-mann's area 44 and the right Brodmann's area 13, fully support this theory.
The present meta-analysis also showed increased likelihoods within the left middle (Brodmann's area 21) and superior temporal gyri (Brodmann's area 22), which compose the associative auditory cortices. The inferior parietal lobule (Brodmann's area 40), part of Wernicke's convolution on the left side of the brain, was also identified as a region of increased activity and is notably involved in speech processing (
83). Interestingly, structural imaging studies report a correlation between the severity of hallucinations and gray matter volume reductions within the left superior (
84,
85) and middle temporal gyri (
86). The potential involvement of these language-related perceptual and motor areas in AVHs is reinforced by research using gyrification and diffusion measures. First, abnormalities in cortical gyrification of the bilateral superior temporal sulci, the left middle frontal sulcus, and the left sylvian fissure (Broca's area) have been seen in chronic hallucinators suffering from schizophrenia, which suggests a neurodevelopmental susceptibility to AVHs in schizophrenia populations (
87). Second, the measured fractional anisotropy within the arcuate fasciculus, a white matter bundle connecting Broca's and Wernicke's regions (
88), was significantly increased in a subgroup of patients experiencing frequent AVHs relative to nonhallucinators (
89,
90), which speaks in favor of a fronto-temporal disconnectivity.
Aside from the language network, other regions of significance were identified by our meta-analytic procedure. Activation of the left hippocampus/parahippocampal region (Brodmann's area 27) was apparent. This region is known to be involved in the formation of new memories about autobiographical events and conscious recollection (
91), and it connects widely distributed association cortices, including the language areas. Moreover, severe damage to this structure usually results in retrograde amnesia, whereas its ictal stimulation may cause experiential hallucinations, such as those experienced during the dreamy state phenomenon (
92). Although abnormalities of this region have been frequently reported in schizophrenia independently of AVHs (
93), some authors have proposed that hippocampal dysfunction might alter dopamine release in the basal ganglia, potentially causing positive psychotic symptoms (
94). Interestingly, other studies investigating cortical activations prior to the onset of AVHs have reported deactivation of the parahippocampal region before symptom onset as opposed to activation during hallucinations (
60,
95). Such parahippocampal deactivation has been shown to be associated with the memory recollection process (
96) and could be involved in the inadequate trigger of activations in language-related areas responsible for the hallucinatory experience. Taken together, these data support models of abnormal remembered episodic memories of speech and suggest the plausible involvement of memory retrieval during AVHs (
6).
The right basal ganglia focus deserves further attention. Strangely, this activation is rarely discussed in the source studies. First, it seems unlikely that this cluster could be related to motor control because more than 80% of the patients involved in studies using a button press paradigm signaled the occurrence of AVHs with their right hand and no complementary activation was measured within the precentral gyrus or the cerebellar cortex. Second, a dysregulation of dopamine systems within thalamo-cortico-striatal circuitry is regularly proposed to account for delusions and hallucinations. However, the medial globus pallidus, which has been shown to be active during AVHs, is devoid of dopaminergic afferents (
97). Finally, the main contributors to this cluster are PET studies (
6,
13). We argue that fMRI might be less sensitive than PET studies for detecting activation within this area. A loss in the blood-oxygen-level-dependent signal could be a consequence of the particular vascular system of the globus pallidus compared with the rest of the capillary bed, or it could be a consequence of an elevated tissue iron level (
97). These data need to be considered in the design of future studies, since explorations of the subcortical structures involved in AVHs move beyond a strict dopamine hypothesis.
Altogether, the findings of the present meta-analysis allow us to discuss the three main pathophysiological -theories of AVHs that were previously mentioned. Our results fully support the following two hypotheses: 1) aberrant activations within sensorimotor cortices and 2) a dysfunction of the verbal memory system during the emergence of AVHs. Therefore, we postulate that abnormal memory retrieval involving the hippocampal/parahippocampal region could trigger dispersed neocortical storage sites, notably those within the language areas, which are responsible for the involuntary emergence of AVHs. Interestingly, in psychotic patients experiencing AVHs, reduced connectivity has been found in neural substrates of episodic verbal memory (hippocampus) and central auditory processing (
98), in accordance with the present meta-analytic findings. A third model suggests that a potential underlying mechanism for AVHs could be a reduced ability to attribute the source of speech (
5,
43,
44,
51,
99,
100). The present review did not find evidence for activations of the cortical midline structures commonly involved in source attribution during AVHs. However, in our view, this does not rule out the mechanism of misperceptions of unbidden thoughts as external speech in hallucinators. Such cognitive dysfunctions in patients with AVHs could be present independent of the hallucinatory state. We believe that further insight in the validation of the misattribution model could be provided by another quantitative review of brain imaging trait studies that compare patients with and without hallucinations during verbal monitoring tasks.
We are aware that this study has some limitations. First, a minimum of 20 to 100 coordinates are usually needed to produce a robust meta-analysis map, depending on the complexity of the underlying cognitive processing. Even though the number of foci included in the present research was substantial (129 foci of interest), we were only able to integrate a modest number of articles and few high-quality studies that did not report stereotaxic coordinates were excluded (
9,
66–68), limiting the power of our analysis to detect more subtle activations. Furthermore, we were not able to control for medication status or patient age across studies as covariates of interest. Incorporation of additional weighting factors for the acquisition methods (magnetic resonance field strengths, etc.) and the intensity scores of activation for all clusters will be incorporated into an upcoming version of the activation likelihood estimation algorithm (
72) and should be considered in future meta-analytic studies of AVHs. Nevertheless, the goal of the present research was to clearly define the spatial localizations of the most frequently replicated activations during AVHs rather than to estimate their magnitudes. Despite the cited shortcomings inherent to the activation likelihood estimation method, our data provide strong evidence for concomitant activations in brain areas involved at different levels of complexity in patients' brain architectures. Thus, they allow us to propose an original view of the pathophysiology of AVHs, integrating previous hypotheses that focus on aberrant activations resulting from disturbed interactions within language and verbal-memory networks. Thus, in our view, the experience of voices can be understood as the combination of distinct mechanisms. First, unbidden auditory memories activate verbal areas of the auditory cortex, making the experience sensory. Then, because the self-tag is missing from these sensory experiences, the phenomenon is experienced as voices. These results also invite new theoretical perspectives because although hyperactivation of the primary sensory cortex (Heschl's gyrus) was identified in some reports (
7,
8,
14), results for this area were not significant after quantitative meta-analysis, and thus the area does not seem necessary for the emergence of AVHs. Even if the present data do not permit drawing conclusions about causality, such a hypothesis is consistent with previous reports about the generation of inner speech or auditory verbal imagery, in which Heschl's gyrus is not activated (
39). We postulate that activation of the primary auditory cortex, sometimes measured during AVHs, might result from the backpropagation of activity in associative cortices. It is possible that increased severity, vividness, or external spatial voice localizations may be related to propagation of such activation to the brain areas directly receiving sensory inputs. Further research will be needed to confirm this last proposal.