Obsessive-compulsive disorder (OCD) is characterized by intrusive obsessions and repetitive compulsions that are time consuming and lead to significant functional impairments (DSM-IV criteria). Twin and family studies suggest a partial genetic etiology of OCD. A review of the OCD twin literature indicates that heritability of obsessive-compulsive symptoms ranges between 45% and 65% in children and 27% and 47% in adults (
1). First-degree relatives of patients are exposed to a fourfold risk of developing OCD symptoms relative to the rates among family members of comparison subjects (
2). The largest genome-wide linkage study revealed evidence for susceptibility loci on chromosomes 3q, 6q, 7p, 1q, and 15q (
3). These latter findings have not been consistently replicated, however.
The failure to replicate the potential genetic underpinnings might be resolved by identifying endophenotypes for OCD (
4). Endophenotypes are quantitative biological or cognitive traits that represent simpler clues to genetic underpinnings than the clinical syndrome itself. According to Gottesman and Gould (
5), endophenotypes are heritable, are associated with the illness, are primarily state-independent, and cosegregate within families and therefore are found in unaffected family members at a higher rate than in the general population. Endophenotypes that fulfill these criteria can either mediate between genes and the clinical phenotype on the causal pathway or be risk indicators that share sets of genes with the clinical phenotype (
6). Candidate endophenotypes of OCD at the neurocognitive level include response inhibition (
7), cognitive flexibility deficits (
7,
8), orbitofrontal dysfunctions during reversal learning (
9), and deficits in planning and working memory (
10) as well as decision making (
8). These functions are altered in patients and in unaffected first-degree relatives. But it remains to be determined whether other criteria for endophenotypes are also fulfilled.
Performance monitoring might represent a very promising candidate endophenotype of OCD. Increased error-related brain activity in patients with OCD has been consistently shown using event-related brain potentials (
11–15) and functional magnetic resonance imaging (
16–18). Furthermore, neuroimaging studies of patients with the disorder have revealed hyperactivity at rest and after symptom provocation in the anterior cingulate cortex, a region that is important for action selection and performance monitoring (
19). Performance monitoring, particularly error monitoring, is typically examined by measuring the error-related negativity (
20,
21). The error-related negativity is a negative deflection at fronto-central electrodes in the response-locked event-related brain potential and peaks shortly after an incorrect response. Source localization and functional neuroimaging studies in nonclinical subjects strongly suggest that the anterior cingulate/posterior medial frontal cortex is one of the principal generators of the error-related negativity and an important structure of an error-processing system (
22,
23). Theories of its functional significance are still debated, but there is some agreement that the error-related negativity can be interpreted as a signal that triggers behavioral adjustment to improve performance and prevent future errors. Several studies have reported a negative-going event-related potential component also following correct responses—the so-called correct-related negativity. Similarities between error-related and correct-related negativity in topography, time course, and assumed source (
24) led to the conclusion that both components reflect overlapping or even identical neuronal and cognitive processes. Since both components show excellent reliability (
25), they are well suited to assess trait characteristics.
In considering the suitability of the error-related negativity as a potential endophenotype, it should fulfill the criteria for endophenotypes (
5). It is associated with OCD in that enhanced error-related negativity amplitudes have been repeatedly found in patients with the disorder (
11–15). A twin study showed substantial heritability, for both the error-related and the correct-related negativity, ranging between 45% and 60% (
26). Furthermore, the enhancement of error-related negativity amplitudes seems to be independent of symptom state in pediatric OCD patients (
14). All these findings point toward the possibility that enhanced error-related brain activity represents an endophenotype for OCD. However, data from unaffected first-degree relatives have not yet been reported.
The purpose of the present study was, therefore, to examine electrocortical indicators of performance monitoring in unaffected first-degree relatives of OCD patients. Together with previous evidence, the hypothesized finding of increased amplitudes of the error-related negativity in unaffected family members would further support the assumption that error-related negativity enhancement qualifies as a candidate endophenotype for OCD.
Method
Participants
Thirty unaffected first-degree relatives of OCD patients, 30 OCD patients, and 30 healthy comparison subjects participated in the present study. All participants were examined by trained clinicians using the Structured Clinical Interview for DSM-IV, had normal or corrected-to-normal vision, reported no history of head trauma or neurological disease, and were aged between 18 and 65 years. Participants were individually matched regarding age, gender, and years of education (
Table 1).
The group of first-degree relatives (parents: N=21; siblings: N=6; offspring: N=3) was recruited via patients with OCD from the outpatient unit of the Humboldt-Universität zu Berlin. First, OCD patients gave written consent for first-degree relatives to be contacted. Then, relatives were asked to participate and underwent diagnostic procedures if they agreed. They were included if at least one first-degree relative had OCD as a primary diagnosis with no comorbid psychotic or substance-related disorders. Relatives were excluded if they had any current axis I disorder or current or past OCD or had received psychopharmacological treatment or psychotherapy. All included relatives were unrelated to each other. Seven relatives were related to participants in the patient group of the present study; the others were not.
Patients were recruited from the outpatient unit. They fulfilled DSM-IV criteria for OCD. All patients received cognitive behavior treatment and were in the initial phase of their treatment. Patients with hoarding symptoms, motor tics, or comorbid lifetime diagnoses of psychotic or substance-related disorders were not included. The majority presented checking symptoms (N=10), washing/contamination symptoms (N=5), or a combination of both (N=7). Others either showed symmetry/ordering symptoms (N=2) or suffered from obsessions (N=6). Fifteen patients had current comorbid diagnoses as follows: affective disorder (major depression [N=4]), anxiety disorder (social phobia [N=3], panic disorder [N=1], generalized anxiety disorder [N=1], specific phobia [N=2]), eating disorder (bulimia nervosa [N=1]), and personality disorder (obsessive-compulsive [N=5], avoidant [N=2], histrionic [N=1]). Ten patients were medicated at the time of the study (selective serotonin reuptake inhibitors [N=7], tricyclic antidepressants [N=3]).
Healthy comparison subjects were recruited through local advertisement. They reported no family history of OCD and no past or present signs of psychiatric disease.
All participants completed the Beck Depression Inventory (BDI) II (
27), the Obsessive-Compulsive Inventory–Revised (
28), and a German vocabulary test measuring verbal intelligence (Wortschatz test [29]). Symptom severity in patients was assessed by a trained clinician using the Yale-Brown Obsessive Compulsive Scale (
30) for OCD symptoms and the Montgomery-Åsberg Depression Rating Scale (
31) for depressive symptoms. Participants received verbal and written information about the aims and procedures of the study and gave written informed consent. Study procedures were in accordance with the ethical guidelines of the Declaration of Helsinki, as approved by the local ethics committee.
Task
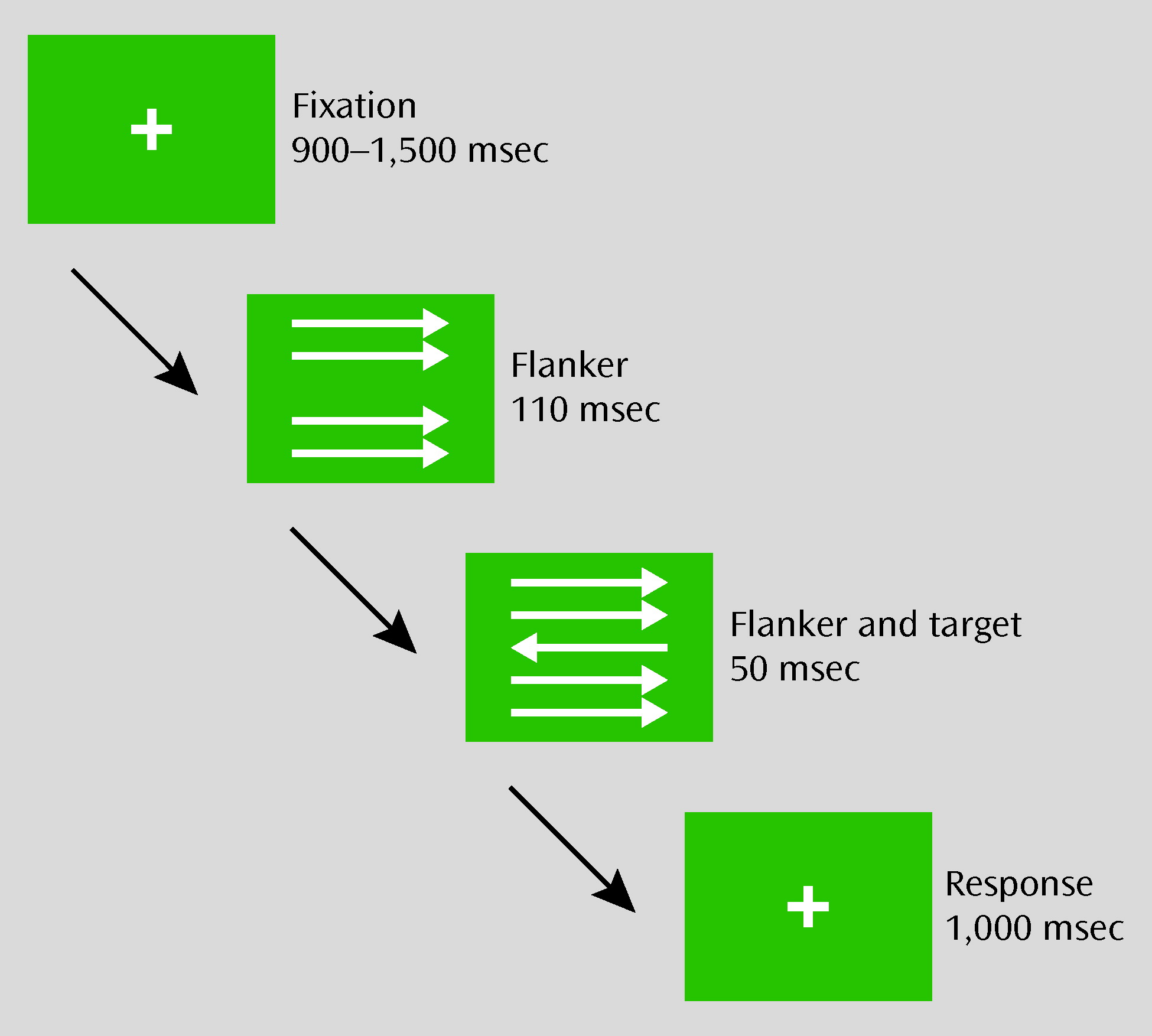
A modified version of the flanker task (
Figure 1) was displayed using Presentation Software (Neurobehavioral Systems, Inc., Albany, Calif.). Participants were instructed to respond with their left or right index finger in accordance with the direction of the target arrow, which was the center arrow in a set of five arrows. At a viewing distance of 65 cm, the set of arrows was 1.2° in width and 1.2° in height. Five hundred trials, including 20 practice trials, were presented. Whereas one-half of the trials were compatible (i.e., the target and flanker stimuli were pointed in the same direction), the remaining were incompatible (i.e., the target and flanker stimuli were pointed in opposite directions). Stimulus compatibility and direction varied pseudorandomly across trials. Every 60 trials, there was a short break, with feedback presented. The feedback instructed participants to respond more accurately when their error rate during the preceding block was >20%. If the error rate ranged between 10% and 20%, participants were reminded to respond both quickly and accurately. When error rates were <10%, they were instructed to respond faster. The total duration of the task was approximately 25 minutes.
EEG Recording, Data Reduction, and Analysis
EEG signals were recorded from 65 electrodes, including Cz as a recording reference. Electrodes were mounted on an electrode cap with equidistant electrode positions (EASYCAP GmbH, Herrsching, Germany). Additional electrodes were placed at the following four locations: IO1, IO2, nasion, and neck. The ground electrode was located below T1. All electrode impedances were <5 kΩ. EEG activity was recorded with a sampling rate of 500 Hz and filtered with a band pass of 0.01–100 Hz. Off-line, the data were re-referenced to the average of all scalp electrodes corrected for eye-movement artifacts using the multiple source eye correction method implemented in BESA 5.1 (Brain Electrical Source Analysis, MEGIS Software GmbH, Gräfelfing, Germany). The continuous EEG signals were digitally filtered with a low-pass filter of 40 Hz and a notch filter of 50 Hz. Response-locked epochs with a duration of 1,200 msec, including a 200-msec preresponse interval, were extracted. Epochs containing voltages exceeding a standard deviation of 100 μV, or voltage steps >40 μV, between consecutive data points were excluded from further analysis. Trials with response times <100 msec and >700 msec were also excluded from averaging. The three study groups did not differ in the number of rejected trials (see Table 1 in the data supplement accompanying the online version of this article). For each participant, averages were computed separately for incorrect and correct responses. The preresponse interval from −200 to 0 msec prior to the response served as a baseline for event-related potentials. Grand averages were filtered with a 15-Hz low-pass filter for visual presentation (
Figure 2), and a Laplace transformation (BESA 5.1) was applied for topographical display (
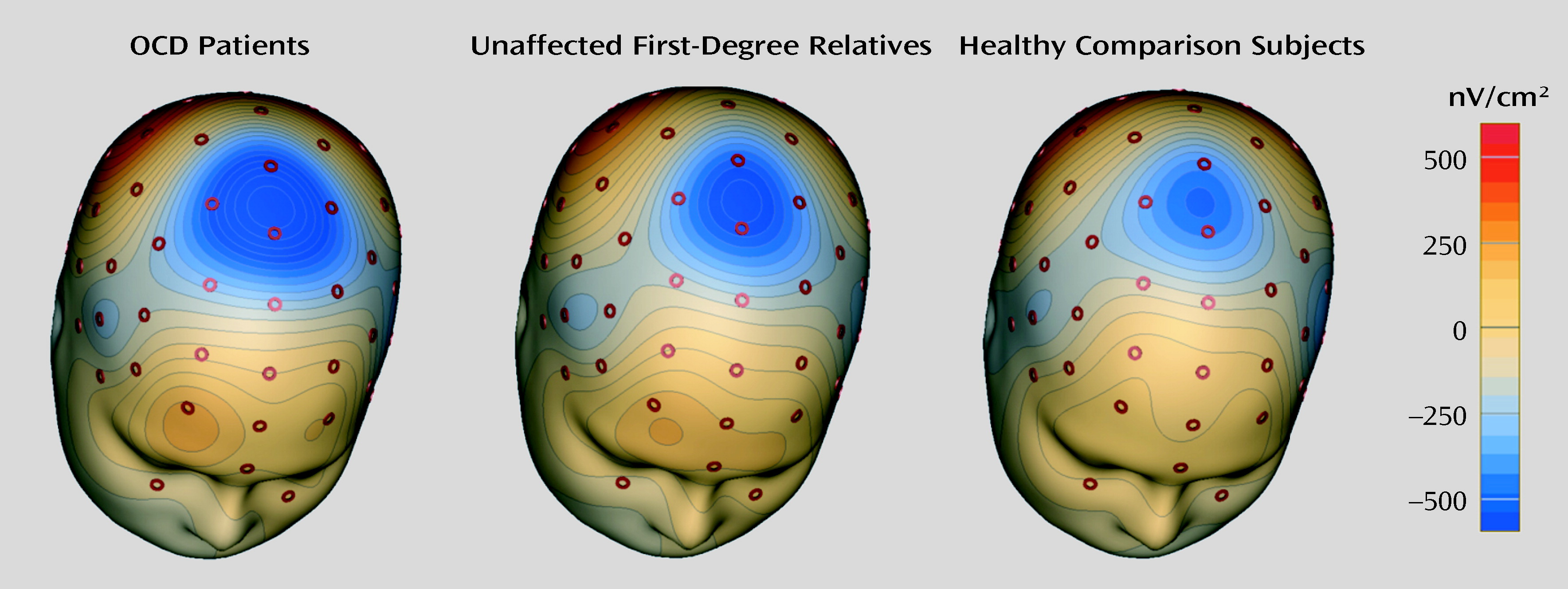
Figure 3). To quantify the response-related negativities, the differences between the most negative peak occurring from 0–150 msec post-response and the immediately preceding positive peak in the 100-msec time window preresponse were computed. Amplitudes were calculated for electrodes Fz, FCz, and Cz.
One-way analyses of variance (ANOVAs) were used to assess whether symptom severity scores and error rates differed between groups. Repeated-measures ANOVAS were used for statistical analyses of response times and psychophysiological measures. EEG measurements were analyzed using group (OCD patients, first-degree relatives, and healthy comparison subjects) as a between-subject factor and electrode (Fz, FCz, and Cz) and response type (correct, error) as within-subject factors. Reaction times were analyzed with group as a between-subject factor and response type as a within-subject factor. Additional analyses for the OCD group consisted of medication status and comorbidity as between-subject factors and electrode and response type as within-subject factors. All statistical tests were two-tailed. Greenhouse-Geisser correction was used for all comparisons with more than two within-subject levels. Post hoc comparisons were corrected using the Bonferroni procedure, and only corrected p values are reported. Correlation coefficients (Pearson's r) were used to examine associations between response-related negativity amplitudes at electrode FCz, symptom severity, and behavioral measures. Statistical analyses were conducted using SPSS, Version 17.0 (SPSS, Inc., Chicago).
Results
Demographic and Behavioral Data
Table 1 shows demographic, clinical, and behavioral measures of OCD patients, unaffected first-degree relatives of OCD patients, and healthy comparison subjects. The groups did not differ in regard to age, verbal IQ, and gender. As expected, the groups differed significantly in regard to symptom severity scores (Obsessive-Compulsive Inventory–Revised: F=51.91, df=2, 87, p<0.001; BDI II: F=12.13, df=2, 87, p<0.001). OCD patients scored higher on both measures than relatives and comparison subjects (p values <0.01). Relatives did not differ from the comparison group on either symptom measure.
The analysis of behavioral measures revealed a group difference in error rates (F=7.62, df=2, 87, p<0.01). OCD patients and relatives showed similar error rates, but both groups had lower error rates than comparison subjects (p values <0.01). Response time data showed a significant main effect of response type (F=1386.68, df=1, 87, p<0.001). Correct responses (mean=357 msec [SD=45]) were significantly slower compared with incorrect responses (mean=261 msec [SD=40], p<0.01). No main effect of group and no interaction between group and response type were found. To analyze posterior slowing, response times in trials following an error were compared with response times in trials following a correct response. To avoid a confoundation with the congruency effect, only response times in incompatible trials were used for computation. The mean difference between posterior and postcorrect response times was 12 msec (SD=33), indicating small but reliable posterior slowing (F=11.12, df=1, 87, p<0.01). The groups did not differ in posterior slowing.
Event-Related Potential Data
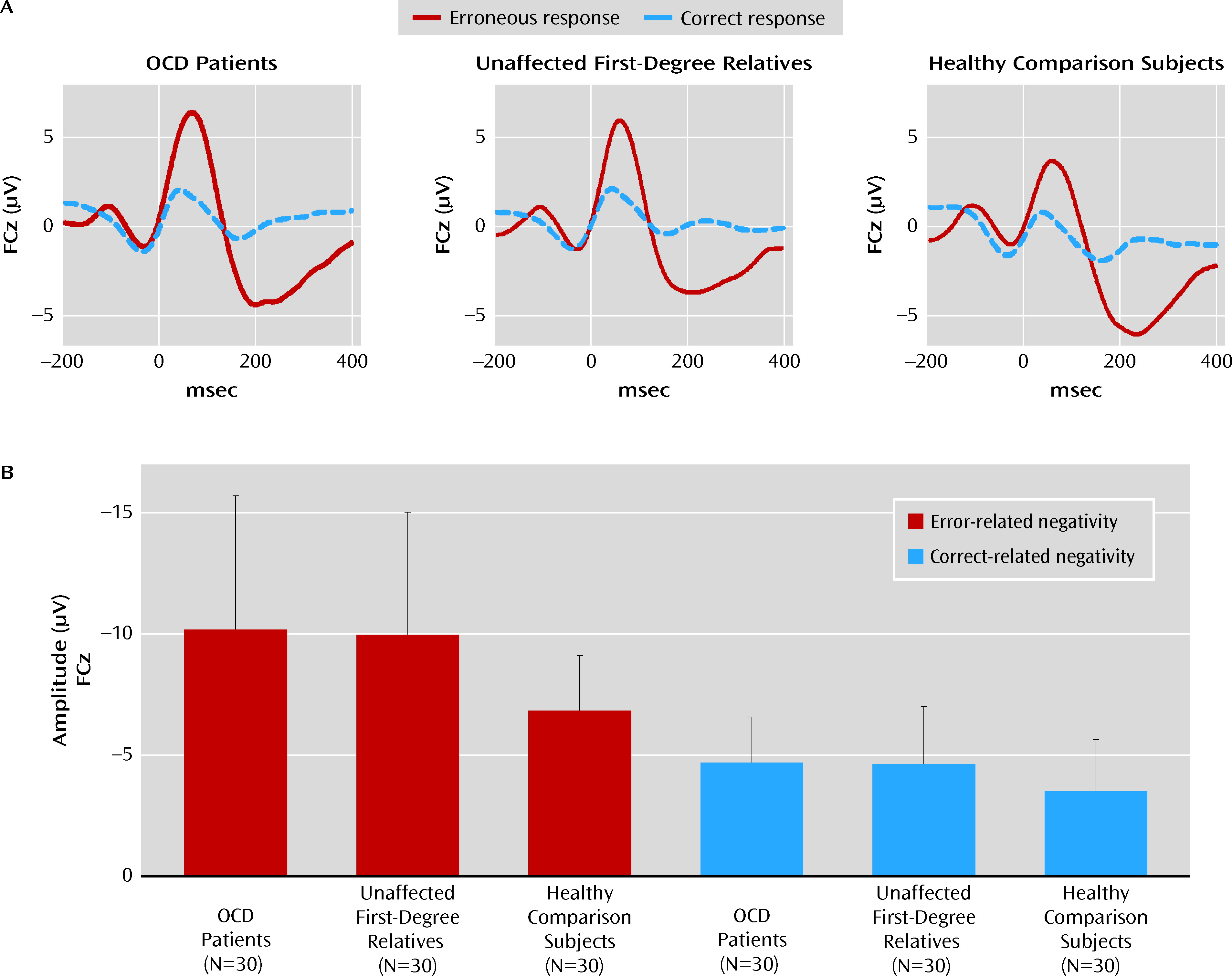
Figure 2 displays grand average waveforms associated with correct and incorrect responses for OCD patients, unaffected first-degree relatives, and healthy comparison subjects. All groups showed more pronounced negativities following errors than following correct responses, reflected in a significant main effect of response type (F=138.41, df=1, 87, p<0.001). A significant main effect of electrode was also observed (F=45.13, df=2, 174, p<0.001; ε=0.67), specified by a significant interaction between electrode and response type (F=83.97, df=2, 174, p<0.001; ε=0.60). Whereas the error-related negativity was largest at electrode FCz and had a clear fronto-central distribution (
Figure 3), the correct-related negativity had a more frontal maximum with similar amplitudes at electrodes FCz and Fz. Most important, there was a significant main effect of group (F=5.95, df=2, 87, p<0.01). Post hoc comparisons revealed enhanced amplitudes in patients and unaffected relatives compared with healthy comparison subjects (p values <0.05). The group main effect was specified by a significant interaction with response type (F=3.15, df=2, 87, p<0.05 [
Figure 2]) as a result of larger group differences for error-related brain activity. Post hoc tests indicated significantly enhanced error-related negativity amplitudes for patients and relatives compared with healthy comparison subjects (patients: p<0.01, Cohen's d=0.82; relatives: p<0.01, Cohen's d=0.79). The magnitude of the error-related negativity did not differ between OCD patients and relatives. Correct-related negativity amplitudes were numerically larger in OCD patients (Cohen's d=0.55) and relatives (Cohen's d=0.45) than in comparison subjects, but these differences did not reach statistical significance. The pattern of results remained stable when amplitudes were corrected for individual differences in error rate and BDI II scores using these variables as covariates in an analysis of covariance (ANCOVA) (main effect group: F=4.36, df=2, 84, p<0.05; interaction group-by-response type: F=4.79, df=2, 84, p<0.05; main effect response type: F=18.14, df=1, 84, p<0.001). To further clarify whether group differences were independent of performance differences, subjects with error rates that differed more than one standard deviation from the total mean were excluded. The remaining subjects (patients [N=26], relatives [N=18], comparison subjects [N=19]) were no longer significantly different in error rates. As in the larger sample, enhanced error-related negativities were found for patients and relatives (main effect group: F=4.72, df=2, 60, p<0.05). Results also remained stable when only patients with washing/contamination symptoms, checking symptoms, or a combination of both were reanalyzed to reduce heterogeneity in the patient group (N=22) (main effect group: F=5.79, df=2, 79, p<0.01).
Additional analyses were conducted to assess whether response-related potentials in patients were influenced by medication or comorbidity. Neither main effects for medication status or comorbidity nor significant interactions were found.
Correlations were computed between error-related negativity amplitudes at electrode FCz, symptom severity scores, and behavioral measures, conducted separately for the three groups. Significant correlations were found in none of the three groups (see Table 3 in the data supplement).
Discussion
The present study examined psychophysiological indicators of performance monitoring in unaffected first-degree relatives of OCD patients and compared them with OCD patients as well as healthy comparison subjects without a family history of OCD. Importantly, unaffected family members of OCD patients showed enhanced error-related negativity amplitudes relative to healthy comparison subjects. In line with previous studies, we found evidence for enhanced error-related negativity amplitudes in OCD patients, reflecting overactive performance monitoring (
11–15). The results of this study further confirm that enhanced error-related brain activity is a promising candidate for a neurocognitive endophenotype of OCD.
Relatives showed enhanced response-related negativities similar to those of OCD patients, although they did not show OCD symptoms and were free of psychotropic medication. Moreover, error- and correct-related negativity amplitudes in the patient group were uncorrelated with symptom severity scores and independent from medication status. These results suggest that overactive performance monitoring reflects a trait marker for OCD that underpins clinical symptoms rather than being the consequence of those symptoms. Together with findings indicating that the magnitude of the error-related negativity is heritable (
26) and state-independent in OCD (
14), the present study further confirms that the enhanced error-related negativity is a candidate endophenotype for the disorder. However, the present data do not allow a decision as to whether enhanced error-related brain activity is a mediator between genes and a phenotype of OCD or only a risk indicator that shares genes with OCD (
6). In addition, endophenotypes might also be influenced by environmental risk factors that affect both the endophenotype and the clinical phenotype (
6). In any case, the identification of endophenotypes is one important step to unravel the etiology of complex psychiatric disorders such as OCD.
Besides the amplitude enhancement of the error-related negativity, larger negativities after correct reactions nearly reached statistical significance. Thus, a process that underlies both components appears to be overactive in OCD, reflecting strategic adjustments (
32) or online response control (
24). The posterior medial frontal/anterior cingulate cortex has been suggested to be one of the principal generators of these components (
22–24). Activity of the anterior cingulate cortex is assumed to trigger the adjustment of cognitive control, which subsequently involves other brain regions (e.g., the prefrontal cortex) to prevent future errors (
22). A persistently overactive performance monitoring system might therefore account for the higher need to control actions and thoughts commonly observed in OCD. According to cognitive models of OCD, it is assumed that this high need for control contributes as a vulnerability factor to the development of the disorder. Recently, it has also been reported that first-degree relatives of OCD patients show higher scores for obsessive beliefs (
33). Although overactive performance monitoring does not predict obsessive-compulsive symptoms, it may interact with other social, psychological, or biological factors in the development of OCD. To further clarify this, it will be important to identify differences between patients and relatives and to investigate these functions with longitudinal designs in subjects who are at risk.
Overactive performance monitoring is also present in healthy individuals with subclinical obsessive-compulsive (
34) and anxiety symptoms (
35), negative affect (
36), and also in patients with major depression (
37). Therefore, it might be better considered as a potential endophenotype for a group of internalizing disorders (
38). There are some hints, however, that error-related brain activity in depression is more affected by symptom state, since it is influenced by induced negative affect (
36) and varies with symptom reduction (
39). In contrast, the error-related negativity enhancement in OCD is independent from symptom state in patients (
14) and was also observed in first-degree relatives. Accordingly, the differential amount of state and trait characteristics reflected by the error-related negativity might vary between depression and OCD. This dissociation might arise from different mechanisms such as a proactive error avoidant and a reactive affective process with distinct contributions from the dorsal and rostral parts of the anterior cingulate cortex (
40). There might also be shared factors among OCD, depression, and other anxiety disorders. Therefore, further studies should examine multiple endophenotypes in groups of OCD, depression, and anxiety disorder patients in order to better understand common as well as distinct processes in the pathogenesis of these disorders.
The present study has several potential limitations. Healthy comparison subjects committed more errors than relatives and patients. This is in contrast with behavioral results of previous studies that reported no performance differences (
11–15). However, the combination of lower error rate and enhanced error-related negativity is in line with the assumption that the error-related negativity signals the need to adjust behavior to improve performance and prevent future errors (
22). The discrepancy in performance between the current study and previous studies might be explained by the use of feedback procedures or different power as a result of sample sizes. Altogether, performance differences in the present study should be interpreted with caution, and future studies are needed to test its robustness. Nevertheless, group differences in error-related brain potentials were confirmed after introducing error rates as a covariate into an ANCOVA and in subsamples that did not differ in error rates. The interpretation of the present results might also be limited by the fact that some patients were medicated, had current comorbid disorders, and were heterogeneous in symptom profiles. However, post hoc tests of these confounds showed that results were not affected by medication or comorbidity in the patient group and also remained stable when controlling for depressive symptoms or after excluding patients with symptoms other than washing/contamination or checking. Most importantly, the central result of enhanced error-related negativity amplitudes in unaffected relatives is not confounded by comorbidity, medication, or therapy status.
In conclusion, this is the first study, to our knowledge, that examined and identified larger error-related negativity amplitudes as an indicator of hyperactive performance monitoring in asymptomatic first-degree relatives of OCD patients. Thus, relatives and OCD patients show similar enhancement in error-related brain activity. The obtained results support the idea that performance monitoring alterations represent a candidate endophenotype of OCD that may indicate a vulnerability to the disorder.