Episodic agitation and aggression are common and significant problems for patients with schizophrenia, putting them at risk for physical and chemical restraint. In a multicenter chart review study of patients in psychiatric emergency settings, 52% were found to have signs of agitation, 25% received medication for agitation, and 6% were physically restrained (
1). In a study of psychiatric inpatients, violent incidents were found to occur at an average rate of 0.51 per week per occupied bed. Substantial morbidity results from aggression and violence on psychiatric units, with 45% of incidents resulting in harm (
2). Both patient factors and environmental factors have been shown to influence the rate of violent behaviors in such settings (
3).
Acute nicotine deprivation and withdrawal in smokers has been shown to increase aggressive behavior (
4), and this effect is more pronounced in individuals with higher baseline irritability or hostility (
5). Patients with schizophrenia have high rates of tobacco use. Across genders, settings, and nationalities, Llerena et al. (
6) found a 78% prevalence of smoking and 58% prevalence of heavy smoking with an odds ratio of 2.6 (95% confidence interval=2.2–3.2) for smoking compared to patients with other mental illnesses. Sensory deficits found in individuals with schizophrenia and their first-degree relatives are transiently corrected by nicotine, and smoking cessation worsens schizophrenia symptoms. Expression of nicotinic receptors is reduced in schizophrenia. Given these findings, it has been suggested that the susceptibility to schizophrenia and to nicotine dependence may be related (
7).
Despite high rates of tobacco use among patients with schizophrenia and the known effects of smoking cessation, little is known about the practical consequences of recent policy changes with regard to smoking in institutions. It is now common in many countries for entire medical campuses to be smoke free. However, partly because of the expense and partly because of patient acceptance, smoking cessation is not a routine part of treatment planning, and nicotine replacement (in the form of gum and patches) is not offered regularly (
3), especially in emergency settings. As a result, forced tobacco abstinence may have the effect of increasing aggressive behavior when patients are confined. Prochaska and colleagues (
3) found that smokers who were not provided nicotine replacement therapy had higher rates of discharge against medical advice, prescription for lorazepam, and use of seclusion than nonsmokers or smokers given nicotine replacement therapy.
Studies in the laboratory have shown that nicotine replacement in the form of nicotine gum can lessen the increase in aggressive behavior resulting from acute tobacco abstinence (
8). Data from other studies suggest that nicotine administration via transdermal patch reduces the frequency of anger reports in both smokers and nonsmokers with high trait hostility (
9). It is possible that nicotine replacement therapy may reduce aggression in both smoking and nonsmoking patients. An attempt was made to test this hypothesis at the University of Colorado hospital, but patients had access to a smoking patio and, despite financial incentives, would not agree to discontinue smoking for 24 hours. The implementation of a smoke-free policy at the Hospital of the University of Geneva (Switzerland) provided an opportunity to perform the study without relying on voluntary abstinence as a condition of study participation.
Method
The study was approved by local and federal ethical committees for the Hospital of the University of Geneva and by the Colorado Multiple Institutional Review Board for the University of Colorado. Participants provided written informed consent after receiving a description of the study and having an opportunity to ask questions.
Participants were 40 smokers with schizophrenia 18–65 years of age who had been admitted to the psychiatric emergency service of the Hospital of the University of Geneva. This service is a separate, dedicated psychiatric emergency service adjacent to a medical emergency department in a general hospital. It has a volume of 6,000 patients per year from the canton of Geneva, with a catchment of 430,000. The service has four holding beds, and patients may remain up to 24 hours. Study participants were diagnosed according to DSM-IV criteria, and the diagnosis was confirmed by an independent psychiatrist using the Mini International Neuropsychiatric Interview.
Assessments
To be eligible for the study, patients had to have a significant level of agitation, as indicated by a score of 14 or greater on the excited component subscale of the Positive and Negative Syndrome Scale (PANSS; 10). The excited component subscale consists of five observational items from the instrument: excitement (P4), hostility (P7), tension (G4), uncooperativeness (G8), and poor impulse control (G14). Investigators were trained in the use of the scale by the first author. Although not developed for or validated in agitated patients, the PANSS is widely used in studies in schizophrenia, and the excited component subscale has been accepted by regulators in the United States and Europe as a primary outcome measure for clinical trials for agitation. This measure has separated drug from placebo in agitated patients with schizophrenia and schizoaffective disorder in trials of olanzapine (
11,
12), aripiprazole (
13,
14), and inhaled loxapine (
15). In addition to its use as a screening measure at baseline, a PANSS excited component subscale rating was also obtained at 4 and 24 hours. Nicotine dependence was assessed with the Fagerström Test for Nicotine Dependence (
16), and a score of at least 6 was required for study eligibility. Patients with major neurologic illness, a developmental disability, a history of other substance abuse within the past month, or a history of significant cardiovascular disease, gastrointestinal disease, or asthma and those who might be pregnant were excluded.
The Overt Aggression Scale (
17), which captures a range of aggressive behaviors, was also used to characterize the study participants and to describe aggressive behavior during the course of the study. In this instrument, violence directed toward self or others is weighted more heavily than verbal assaults or aggression directed at property or animals. The initial rating was for the preceding 7 days, and the study ratings were for 24 hours, so it is used here for descriptive purposes only.
The primary outcome measure was Agitated Behavior Scale (
18) score. This 14-item instrument has been validated in multiple brain-injury and psychiatric populations and used as a secondary outcome measure in some similar trials. The use of different measures to assess agitation for study inclusion and for outcome was designed to minimize any possible effect of inflation on baseline ratings for inclusion purposes.
The Richmond Agitation-Sedation Scale, which was developed in medical settings, defines normal as zero and measures agitation with positive scores and increasing sedation with negative scores (
19). It was used in this study primarily as a measure of sedation.
After screening and administration of baseline measures, participants were randomly assigned to receive either nicotine replacement therapy with a 21-mg transdermal patch (N=20) or a placebo patch (N=20), according to a randomization table generated in Excel. The patch was placed by a nurse, and both patient and investigator were blind to the treatment. Patients were confined in a secure area and regularly checked by nursing staff to ensure abstinence. Other than these patches, participants received usual care. Those who were already receiving appropriate treatment continued the treatment unchanged. Those who were not already receiving treatment or were not receiving adequate treatment received 5 or 10 mg of olanzapine orally or intramuscularly or 5 mg of haloperidol intramuscularly, according to the service's written guidelines.
Analysis
Descriptive statistics were computed for age, sex, and clinical variables. Chi-square tests and t tests were used to compare baseline patient characteristics between the nicotine replacement and placebo groups. The primary outcome measure was agitation at baseline and at 4 and 24 hours, as assessed by the Agitated Behavior Scale. The secondary outcome measure was agitation at the same time points, as assessed by the PANSS excited component subscale.
General linear mixed-effects models were used to compare agitation scores over time for patients in the intervention and placebo groups. Repeated measures within patients were modeled with time coded as a categorical variable to facilitate comparisons over the course of treatment. The primary effect of interest was change in agitation scores from baseline for the placebo group compared with the intervention group, assessed by means of linear contrasts defined a priori. Data were complete for all 40 patients. Covariates were included if they were significantly associated with the outcome or differed between the treatment groups. A three-way interaction between baseline Fagerström score, treatment group, and time was added to the model to assess for differential effects of treatment on agitation in patients with high (>8) versus low (ranging from 6 to 8) scores for nicotine addiction. Additional analysis tested whether nicotine addiction levels moderated the intervention effect—that is, whether change over time differed in the two groups for high compared with low nicotine addiction (
20). All analyses were conducted with SAS, version 9.2 (
21).
Results
Baseline agitation scores on the excited component subscale of the PANSS ranged from 14 (the minimum for study eligibility) to 28 (mean=20.7, SD=2.72). No eligibility cutoff was set for Agitated Behavior Scale score, so if baseline inflation of the PANSS excited component subscale score had occurred, the distribution of baseline scores on the two instruments would be expected to differ. However, Agitated Behavior Scale scores were also truncated, ranging from 20 to 48 (mean=28.93, SD=5.22). Both sets of scores were normally distributed and were highly correlated (Pearson's r=0.82, p<0.001).
There were no significant differences between groups in age, sex, baseline nicotine dependence, or baseline agitation as measured by the PANSS excited component subscale, the Agitated Behavior Scale, or the Richmond Agitation-Sedation Scale. However, nine patients in the nicotine group had a history of aggression in the past week, compared with only two in the placebo group, resulting in significantly higher baseline Overt Aggression Scale scores in the nicotine group (p=0.03).
Overall change in Agitated Behavior Scale scores in the intervention compared with the placebo group approached statistical significance in mixed-effects models (F=3.02, df=2, 76, p=0.055) after controlling for baseline scores on the Overt Aggression Scale and the Fagerström test. Age and sex were not significantly associated with Agitated Behavior Scale score and were not included as covariates. Agitation diminished over time in patients in both the intervention and placebo groups. In the placebo group, the mean reduction from baseline in Agitated Behavior Scale score was 30.5% at 4 hours (8.75 points, p<0.001) and 39.0% at 24 hours (11.2 points, p<0.001). In the intervention group, the mean reduction in score was 40.9% at 4 hours (11.9 points, p<0.001) and 48.1% at 24 hours (14 points, p<0.001). Linear contrasts were used to examine differences in reduction between placebo and intervention patients at 4 hours (3.15 points, p=0.028) and at 24 hours (2.8 points, p=0.050). The intervention group had a 33% greater reduction in agitation at 4 hours and a 23% greater reduction at 24 hours.
In the analysis with PANSS excited component subscale score as the outcome measure, change over time differed significantly for the intervention group compared with the placebo group (F=4.85, df=2, 76, p=0.01). Although agitation in both groups improved, the intervention group had a greater reduction than the placebo group at 4 hours (2.1 points, p=0.006) and at 24 hours (1.85 points, p=0.014).
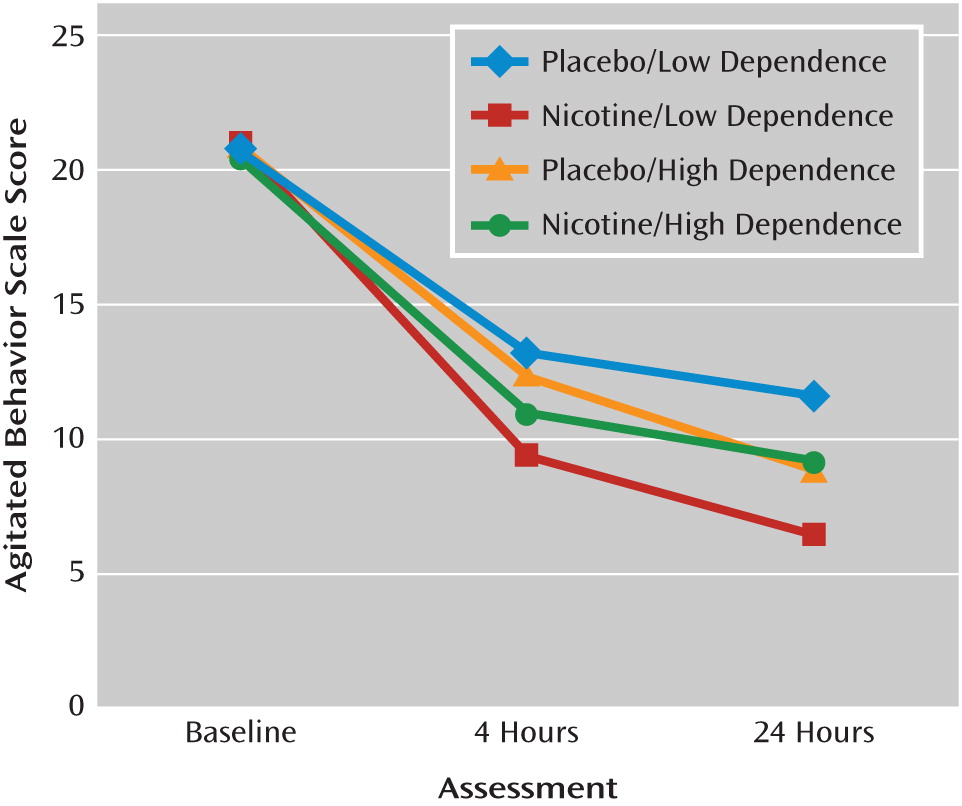
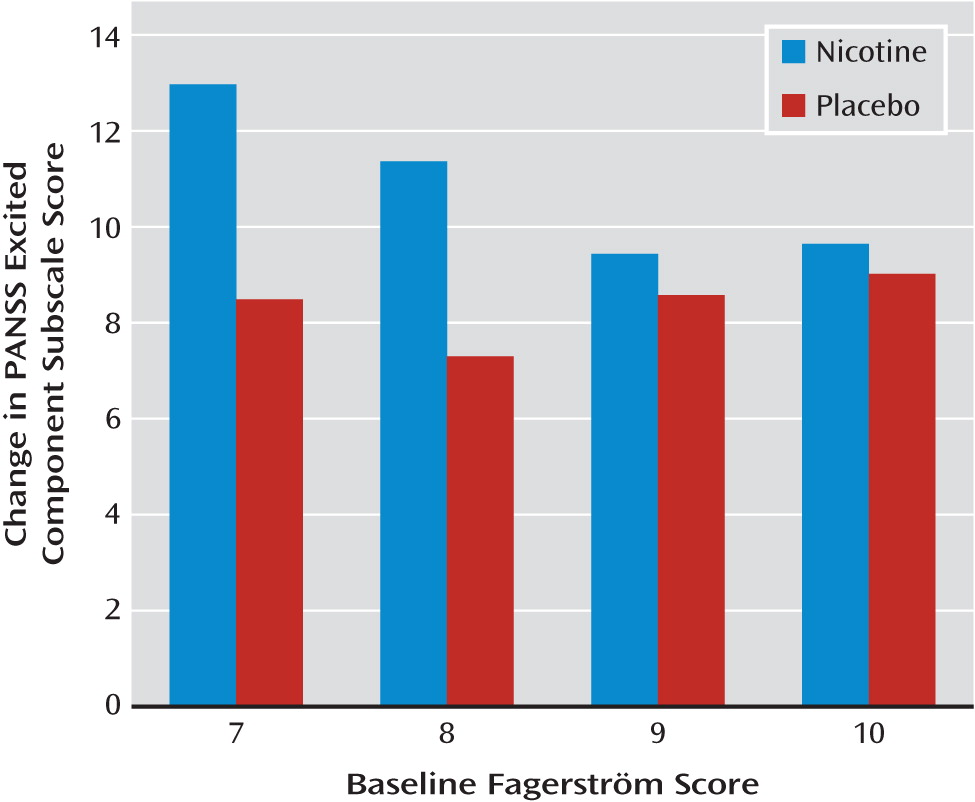
Additional analyses were carried out to determine whether baseline Fagerström scores moderated patients' response to nicotine replacement. The addition of a three-way interaction term to the model with Agitated Behavior Scale score as the outcome variable indicated that lower Fagerström score at baseline was associated with better response to nicotine replacement therapy (F=4.28, df=2, 72, p=0.018). For patients with Fagerström scores of 7 or 8 (low dependence), there was a significant difference in reduction between nicotine and placebo groups at 4 hours (t=2.74, p=0.010) and at 24 hours (t=3.08, p=0.004). However, patients with Fagerström scores of 9 or 10 (high dependence) had a similar reduction at 4 hours (t=1.39, p=0.173) and at 24 hours (t=0.53, p=0.596). Although a Fagerström score of 6 met the inclusion criteria, none of the patients in the study had a score below 7. The addition of a three-way interaction term to the model with Agitated Behavior Scale score as the outcome variable indicated that lower Fagerström score at baseline was associated with better response to nicotine replacement therapy (F=4.28, df=2, 72, p=0.018) (
Figure 1). Results for the excited component subscale of the PANSS were similar (F=44.60, df=11, 108, p<0.0001);for patients with Fagerström scores of 7 or 8 (low dependence), there was a significant difference in reduction between nicotine and placebo at 4 hours and at 24 hours. However, patients with Fagerström scores of 9 or 10 (high dependence) had a similar reduction at 4 hours and at 24 hours (
Figure 2).
According to the Richmond Agitation-Sedation Scale, six patients in the nicotine group were drowsy to moderately sedated but aroused with verbal stimuli at 4 hours, and three in the placebo group became drowsy. No patient was unarousable at any point. Three patients were aggressive during the study, but there were no serious or unexpected adverse events in either group.
Discussion
Despite high rates of tobacco use among patients with schizophrenia, little is known about the acute effects of abstinence or access to nicotine replacement therapy in this population, especially in emergency settings. This is the first randomized study designed to test the effect of nicotine replacement therapy on agitation and aggression in smokers with schizophrenia.
We used one agitation measure to define the population and another to measure change, and the high correlation between them suggests that the measures are valid. The baseline rating of aggression in this study was self-reported and based on the past 7 days, whereas ratings for the treatment phase were prospective and observer rated, but ratings were made for only 24 hours, and most participants were not aggressive at all. Thus, we did not compare participants on the Overt Aggression Scale. However, 11 of the 40 participants (28%) reported aggressive behavior during the week preceding the study, and three (8%) displayed aggressive behavior during the study despite usual treatment with antipsychotics. Questions have been raised as to whether participants in clinical trials in agitation are representative of those in practice. The level of aggression reported here, however, suggests greater external validity.
Randomization produced comparable groups with no significant differences in age, sex, degree of nicotine dependence, or baseline level of agitation. The levels of agitation reported here were moderate to severe and similar to those reported in other studies of agitation in which patients consented to participate (
11). With lower levels of agitation, it may be more difficult to demonstrate differences between treatments. Likewise, treatment with antipsychotics might be expected to reduce the power to detect differences between the nicotine replacement therapy and placebo groups. However, despite the moderate level of agitation and the use of usual treatment with antipsychotics in this study, our data indicate that patients receiving nicotine replacement therapy had significantly lower levels of agitation 4 and 24 hours after baseline. The effect size is small, but the magnitude of the drug-placebo difference is similar to that observed in industry trials of parenteral antipsychotics (
10). Given the frequency and hazards of physical restraint and the limitations of available treatments (
22), nicotine replacement therapy could have a significant effect on the care of agitated patients.
The fact that patients with lower Fagerström scores were significantly more responsive to nicotine is consistent with a dose effect. It is possible that the 21-mg patch was inadequate for the more nicotine-dependent individuals in this study. The maximum serum concentration (Cmax) of nicotine occurs at approximately 6 hours rather than 4 hours, but a substantial proportion of Cmax is present within 4 hours. We chose to study transdermal delivery of nicotine for its predictability, but nicotine gum should also be effective and has a rapid onset. It may be necessary to combine gum with the patch or otherwise obtain higher doses to better manage agitation in this patient population.
These findings could be related to several factors that deserve further study. In addition to the level of nicotine dependence, it possible that trait or state irritability or hostility may be important, as it is in nonpatient samples (
8,
9). It is also possible that addiction and withdrawal are not required for nicotine to have an amelioratory effect on agitation. If so, use of nicotine would need to be balanced against the greater likelihood of adverse effects in nicotine-naive or nondependent patients. It would also be of interest to examine the relationship of P50 evoked potential, galvanic skin conductance, and other biomarkers of agitation in this and other populations.
This study has several limitations. It should be replicated in a larger sample, preferably at multiple sites and with multiple, larger fixed doses of nicotine and a longer observation period. Higher levels of agitation are observed in studies of patients who have not provided consent. In our study, consent was obtained and some screening was required prior to study entry, limiting the extent to which the most agitated patients could be included. Participants also received antipsychotic medications, making this effectively a study of the combination of antipsychotics and nicotine replacement therapy compared with antipsychotics and placebo. Although allowing patients to continue their existing treatment or use one of two protocol-defined treatments may introduce some variance, limiting participants to a single antipsychotic at a fixed dose and thus changing some patients' antipsychotic treatment would unnecessarily raise practical and ethical issues. Allowing a variety of antipsychotics increases the ecological validity of the study, given that few physicians would advocate treatment with nicotine alone in this population, and it poses one less obstacle to patient acceptance.
Our results suggest that nicotine replacement in addition to usual care reduces agitation in smokers hospitalized for schizophrenia by about one-third. Attention to smoking history and nicotine replacement where indicated may be beneficial and should certainly be considered in patients at risk for physical restraint or forced treatment.
Acknowledgments
The authors acknowledge the assistance of Jonathan Berry, M.D., with early drafts of the research proposal.