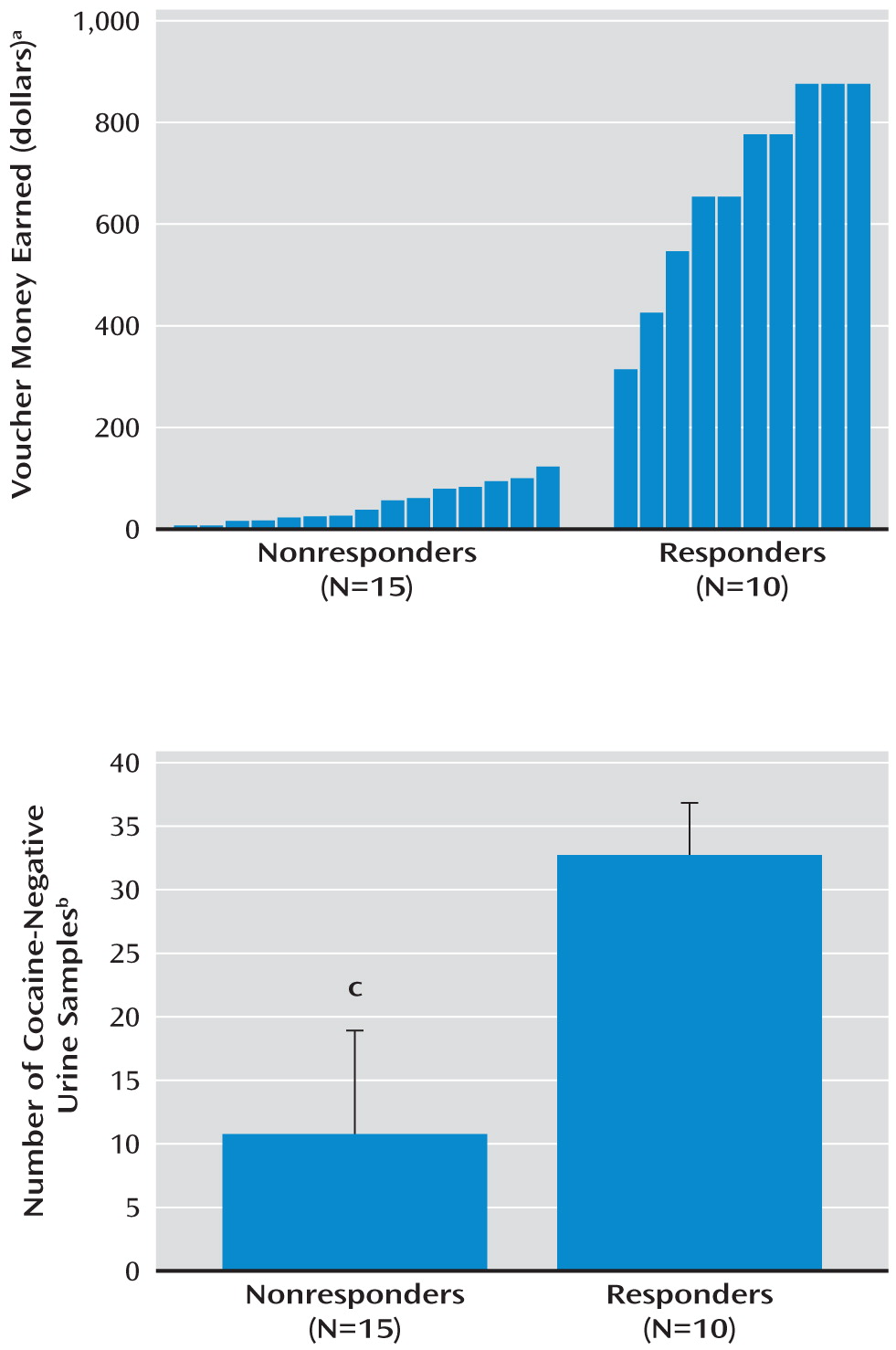
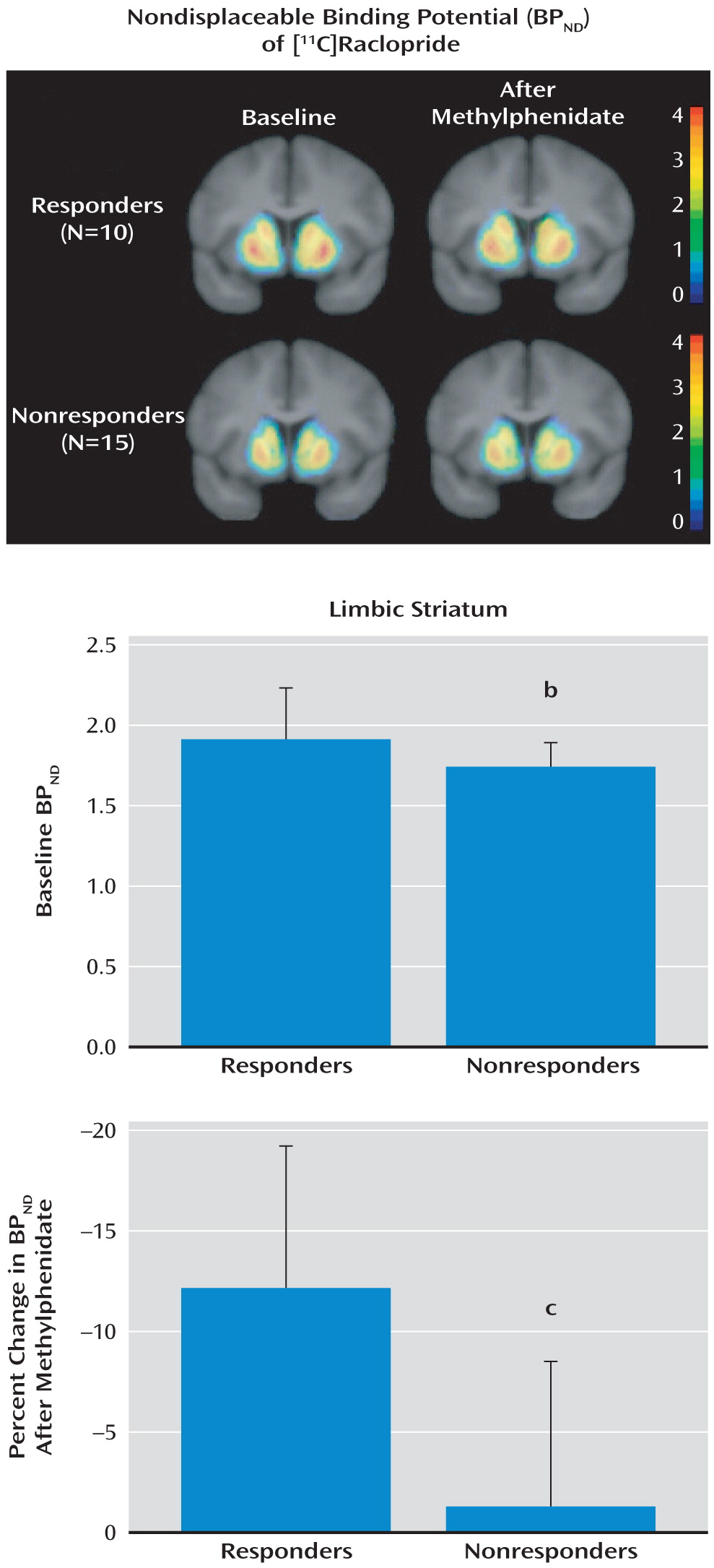
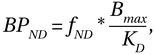The role of dopamine in the striatum is among the most studied phenomena of the brain. For almost a half century, it has been shown that striatal dopamine is a crucial component of reward, reward-based learning, and addiction (
3,
4). The nucleus accumbens, which is contained within the limbic striatum in humans, serves as a hub of the brain's reward pathways, and dopamine transmission in this brain region plays a central role in selecting adaptive, motivated behavior (
5). Positron emission tomography (PET) imaging with the radioligand [
11C]raclopride is frequently used to provide quantitative information about striatal dopamine type 2 and type 3 (D
2/3) receptors. In addition to measuring D
2/3 receptors, this radiotracer is sensitive to fluctuations in endogenous dopamine (
6,
7). The administration of a psychostimulant, such as methylphen-idate, blocks the dopamine transporter and prevents dopamine reuptake from the synapse, which then increases extracellular dopamine. In the setting of increased dopamine levels, imaging with [
11C]raclopride results in lower radioligand binding, since fewer D
2/3 receptors are available to bind to the radiotracer (
6,
8).
Using these methods, investigators have shown that both baseline D
2/3 receptor binding and stimulant-induced dopamine release are lower in cocaine-dependent subjects than in healthy comparison subjects (
9,
10). Our group previously investigated the relationship between dopamine release and a laboratory model of cocaine-seeking behavior (
10). In that study, PET scans of non-treatment-seeking cocaine-dependent human volunteers were followed by cocaine self-administration sessions. In these sessions, the participants chose between low-dose smoked cocaine and an alternative positive reinforcer (money). The results showed that cocaine abusers with low stimulant-induced dopamine release (measured as the change in [
11C]raclopride binding potential) in the limbic striatum were more likely to choose cocaine over money, and they suggest that low dopamine release is associated with compulsive cocaine use (
10).
The goal of the present study was to investigate whether this finding from the laboratory applies to the real-world treatment clinic. Treatment-seeking cocaine-dependent subjects underwent PET scans using [
11C]raclopride to provide two parameters associated with dopamine transmission: 1) baseline dopamine D
2/3 receptor binding, measured as nondisplaceable binding potential (BP
ND) (described in the Method section), and 2) stimulant-induced presynaptic dopamine release, measured as the stimulant-induced change in BP
ND. Following the scans, the subjects were enrolled in treatment consisting of contingency management combined with the community reinforcement approach developed by Higgins et al. (
11,
12). This treatment uses positive reinforcement (monetary vouchers) to induce abstinence from cocaine, which is similar to the choice presented in the laboratory in our previous study (
10). Since the results of our previous study showed that the subjects who chose cocaine use over money had low presynaptic dopamine release (change in BP
ND) in the limbic striatum, we hypothesized that treatment-seeking subjects who did not respond to a treatment that uses a monetary reward to reduce cocaine use would also have low dopamine release (change in BP
ND) in the limbic striatum. In addition, since previous studies in animals have shown that low D
2/3 receptor binding potential (BP
ND) is associated with greater cocaine self-administration (
13,
14), we hypothesized that subjects who did not respond to treatment would also have low dopamine receptor binding potential in the limbic striatum.
A group of comparison subjects was also included in order to show that this cohort of cocaine-dependent subjects had the same abnormalities in neurochemistry reported in previous studies (
9,
10,
15,
16). In addition, the cocaine-dependent subjects were asked to return for follow-up PET scans at the end of treatment (12 weeks) in order to assess the effect of treatment on dopamine transmission. Our hypothesis was that subjects who responded to treatment would show normalization (i.e., increases) in both baseline D
2/3 receptor binding potential (BP
ND) and presynaptic dopamine release (change in BP
ND) in relation to their pretreatment scans.
Discussion
The results of this study show that response to a behavioral treatment for cocaine dependence is related to dopamine signaling in the limbic striatum, measured with PET as D2/3 receptor binding (BPND) and presynaptic dopamine release (change in BPND after methylphenidate administration). The cocaine-dependent subjects who responded to a behavioral treatment that uses positive reinforcement and psychotherapy had higher D2/3 receptor binding and dopamine release than did subjects who experienced relapse in this treatment setting.
Animal studies have previously shown that deficits in dopamine signaling in the nucleus accumbens impair operant conditioning, response inhibition, and behavioral flexibility with respect to reinforced behavior (
26). Creating lesions in the nucleus accumbens of rodents results in a profound deficit in the animals' ability to choose appropriately between two reinforcers: they impulsively and consistently chose a lesser reward over a delayed reinforcer of greater value (
27). These findings suggest that dopamine signaling in the limbic striatum is critical for making the shift between competing reinforcers, such that in the setting of low dopamine transmission a habitual behavior is produced even in the presence of an alternative reward of greater value. We have demonstrated a similar finding in human cocaine abusers. In two cohorts of cocaine-dependent volunteers, some not seeking treatment (
10) and some who were seeking treatment (reported here), low dopamine release in the limbic striatum was associated with the choice to consume cocaine over alternative reinforcers. In each case, the subjects with low dopamine transmission made the nonadaptive choice between competing rewards. Our previous study in the laboratory gave subjects a choice between a low dose of cocaine (6 mg) and $5, and the choice was weighted toward the money, since the street value of this dose of cocaine was less than $5. In the present study, subjects came to the clinic in search of treatment and could earn money for pursuing their goal. Therefore, in both the nontreatment and treatment studies, the more adaptive response was to choose money and abstinence over cocaine, yet in both studies there were a number of subjects who reliably chose cocaine. The failure of the cocaine-dependent subjects with low dopamine release to alter their behavior can be viewed as a perseverative error in the setting of competing rewards or as a blunted brain reward system that is unable to respond to alternative sources of reward.
Ultimately, the question is whether PET radioligand imaging in human cocaine abusers can be used to guide the development of better treatment. Imaging studies have consistently shown that dopamine transmission is blunted in cocaine-dependent subjects, relative to comparison subjects, as indicated by four different findings: 1) lower baseline D
2/3 receptor binding (BP
ND) of the postsynaptic neurons (
9,
10,
15,
16), 2) lower presynaptic dopamine release (change in BP
ND) (
9,
10), 3) lower presynaptic neuronal stores of dopamine (
28), and 4) lower baseline levels of endogenous dopamine (
29). In the present study we investigated the association between dopamine transmission and response to treatment, and these results show that a positive response is associated with higher D
2/3 receptor binding and greater methylphenidate-induced dopamine release than occur in those for whom treatment fails. These findings suggest that increasing striatal dopamine transmission would be the most appropriate strategy for converting treatment nonresponders to responders, by either increasing D
2/3 receptors or increasing presynaptic dopamine. Previous studies in rodents have shown that using a viral vector to increase striatal D
2 receptors reduces the animals' preference for drugs of abuse (
14,
30). On the basis of those data and the findings from the present study, it can be surmised that increasing D
2/3 receptors would improve treatment response, but this technology is unlikely to translate into human use in the near future.
Another approach is to increase presynaptic dopamine release. A number of previous clinical trials have investigated medications that increase striatal dopamine transmission, and while some showed success, others did not (
31). One reason for this inconsistency may be that medications that are known to increase dopamine transmission in the nonaddicted brain may have a minimal effect in the addicted brain, as shown by this study. Notably, Schmitz et al. (
32) reported that treatment of cocaine abusers with contingency management and
l-dopa/carbidopa, which would be expected to improve dopamine transmission by increasing presynaptic stores in the striatum, resulted in a greater response to treatment than occurred with placebo. Another approach may be to increase dopamine transmission by targeting other receptor systems, such as the kappa or acetylcholine receptors (for reviews, see references
33 and
34). Together, these findings strongly suggest that the combination of pharmacology addressing the deficit in dopamine transmission combined with a behavioral treatment that presents tangible alternatives to cocaine use may provide the best approach for the treatment of cocaine addiction.
This study also examined the effect of treatment on dopamine receptor binding and presynaptic dopamine release. No effect of treatment was seen in the nine treatment responders who were scanned before and after treatment, contrary to our hypothesis. However, it is interesting that the treatment responders did not differ from the comparison subjects before treatment, suggesting that presynaptic dopamine was largely intact in the responders to begin with. Among the nonresponders, only six returned for scans after 3 months, and there was also no change in dopamine receptor binding or dopamine release, which is expected since these subjects had continued their cocaine use.
Previous studies using functional MRI have investigated the correlation between brain activation and treatment response (
35,
36). Kosten et al. (
35) showed that low treatment effectiveness correlated with greater cue-induced activation of sensory, motor, and limbic cortical areas, while Moeller et al. (
36) used a working memory task to show that cocaine-dependent subjects with low thalamic activation had a poor response to treatment. A limitation of PET imaging with [
11C]raclopride is that our investigations are limited to the striatum, and other brain regions are also likely to play a critical role in the human condition (for a review, see reference
37). However, imaging with [
11C]raclopride allows a more direct investigation of the aberration in chemistry that occurs with drug addiction, which may provide more guidance in the selection of candidate medications.
On the basis of previous studies in both animals and humans showing that the limbic striatum is most directly involved in reward-related behaviors, we limited our initial analysis to the limbic striatum. With this constraint, both BP
ND and change in BP
ND were significantly lower in the nonresponders. However, if we had used correction for multiple observations (which would have been necessary if our hypothesis had included all regions), only the finding for change in BP
ND would have reached significance. It is interesting that in our previous study (
10) we saw no correlation between the choice to self-administer cocaine and BP
ND, which suggests that the BP
ND effect is less than the effect of change in BP
ND. Another limitation of this study is that the left and right regions were averaged and not analyzed individually, such that there could have been an effect of laterality that we did not see. In addition, while the stimulant-induced decrease in [
11C]raclopride binding correlates with presynaptic dopamine release (
6), studies have shown that receptor internalization or dimerization plays a key role (
7,
38,
39).
In conclusion, the findings from this study were as follows: 1) relative to comparison subjects, cocaine-dependent subjects had blunted striatal dopamine signaling, 2) within the cocaine-dependent subjects, a positive response to treatment was associated with greater dopamine signaling, and 3) treatment itself did not change dopamine transmission. These findings, combined with data from previous studies, suggest that improving dopamine transmission may be the most appropriate treatment strategy for cocaine-dependent subjects who seek treatment but relapse nonetheless.