Almost all established antidepressants target the monoamine systems (
1). However, full and partial resistance to these drugs and their delayed onset of action suggest that dysfunctions of monoaminergic neurotransmitter systems found in major depressive disorder represent the downstream effects of other, more primary abnormalities. Several lines of evidence suggest a glutamatergic dysfunction as a primary abnormality in depression. A single dose of ketamine, a glutamate
N-methyl-
d-aspartate (NMDA) receptor antagonist, has been shown to produce rapid and large antidepressant effects in patients with treatment-resistant depression (
2). Inhibitors of glutamate release (e.g., lamotrigine, riluzole) have antidepressant properties (
3). Abnormal glutamate levels were found in depressed patients in MR spectroscopy (
4). Finally, there is evidence for abnormal NMDA signaling in postmortem tissue preparations in major depression (
5).
Metabotropic glutamate receptors are known to regulate glutamate neurotransmission and interact with monoamine neurotransmitters that are involved in the neurobiology of depression (
6). The metabotropic glutamate receptor 5 (mGluR5) has been proposed as an attractive target for modulating glutamatergic neurotransmission (
7) because it is present not only at postsynaptic neurons but also on glia cells, where it appears to modulate the stimulation of extrasynaptic NMDA receptors (
8). In rats, prolonged blockade of mGluR5 exerts strong anxiolytic- and antidepressant-like effects (
9), and mGluR5 knockout mice express an antidepressant-like phenotype (
10).
Discussion
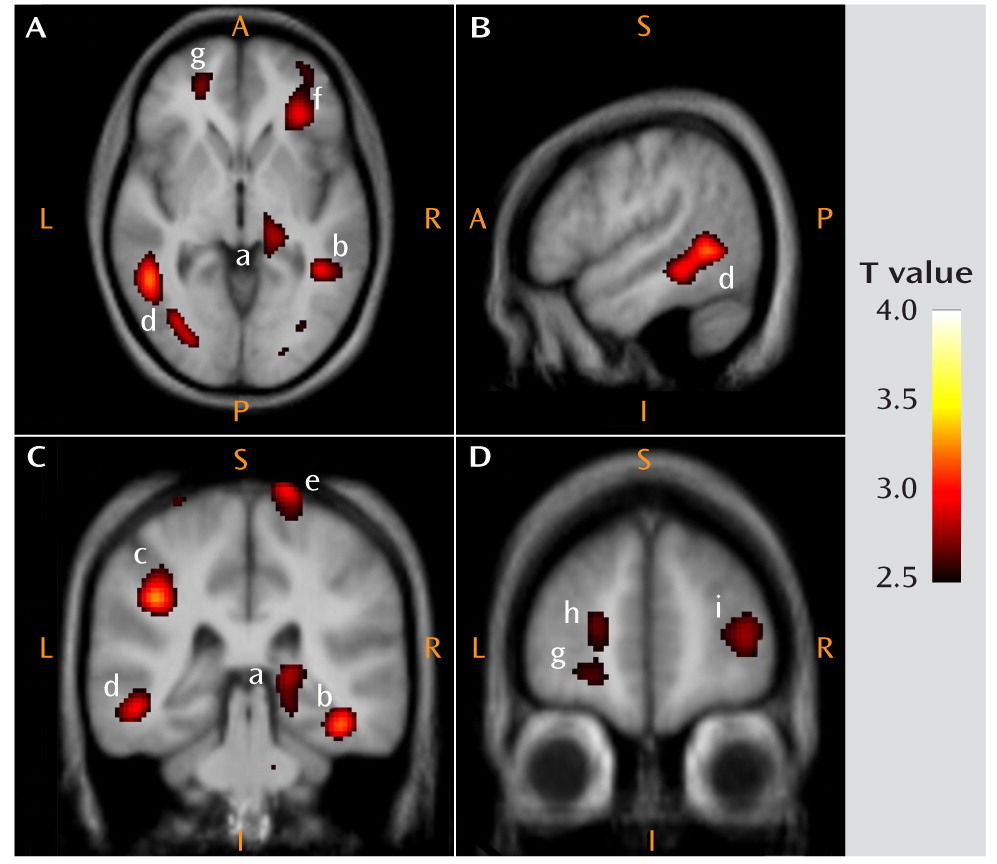
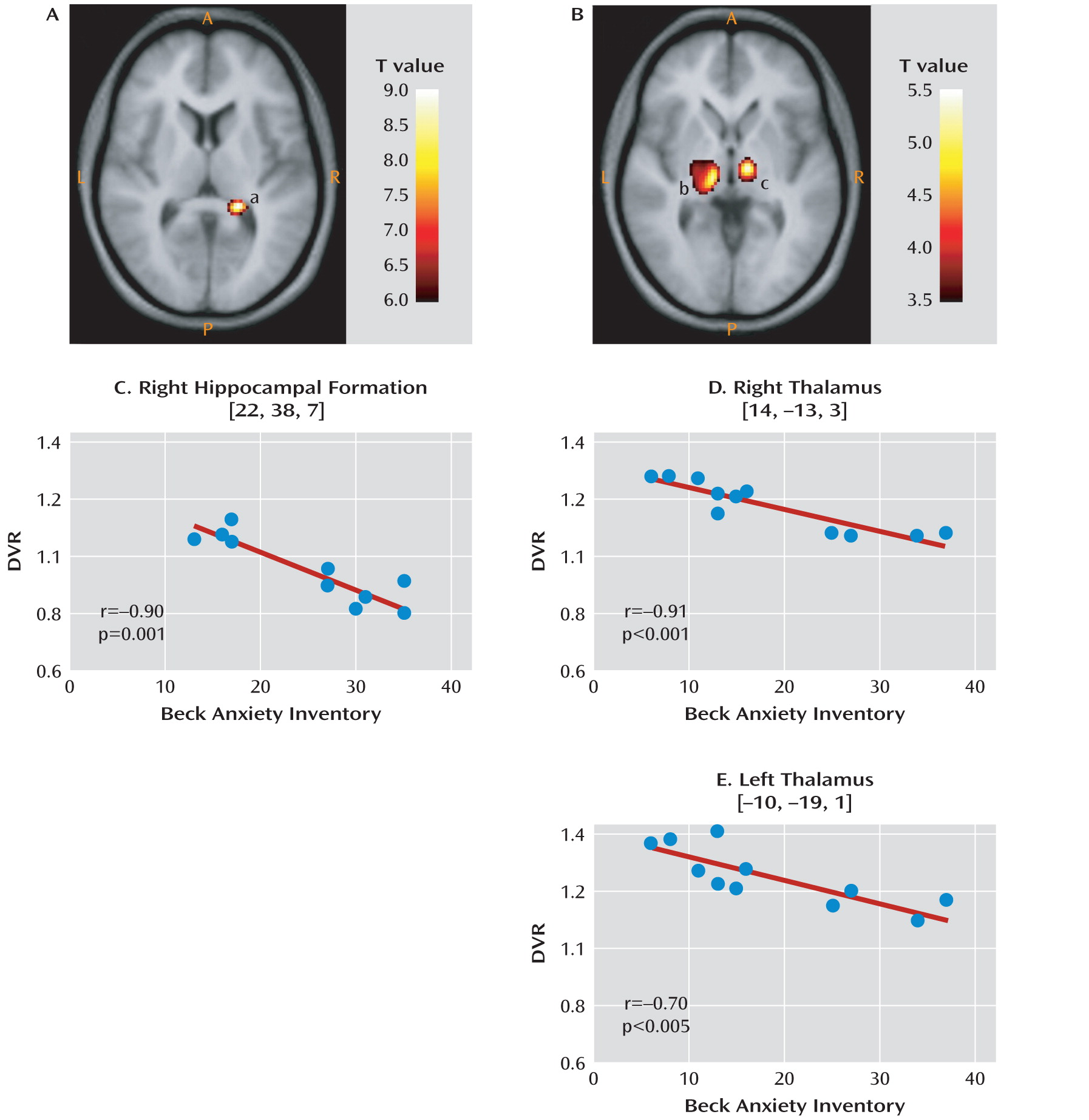
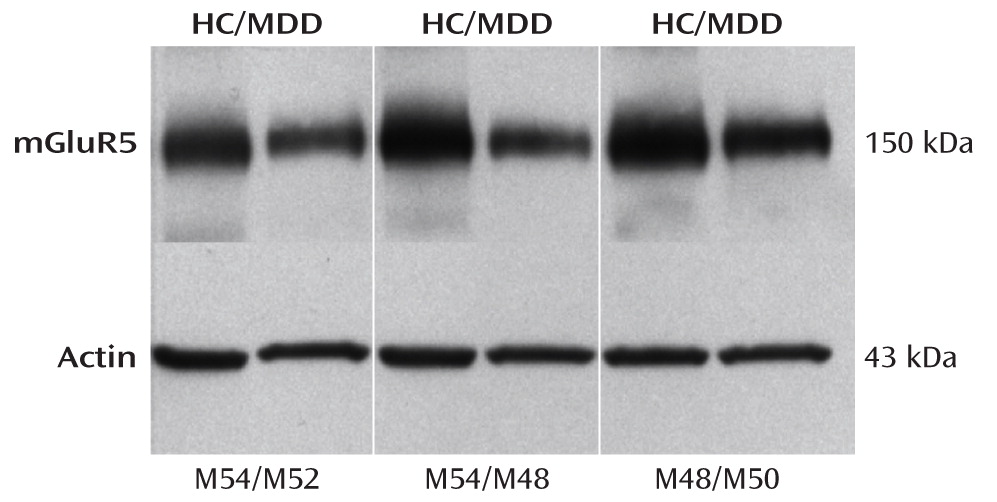
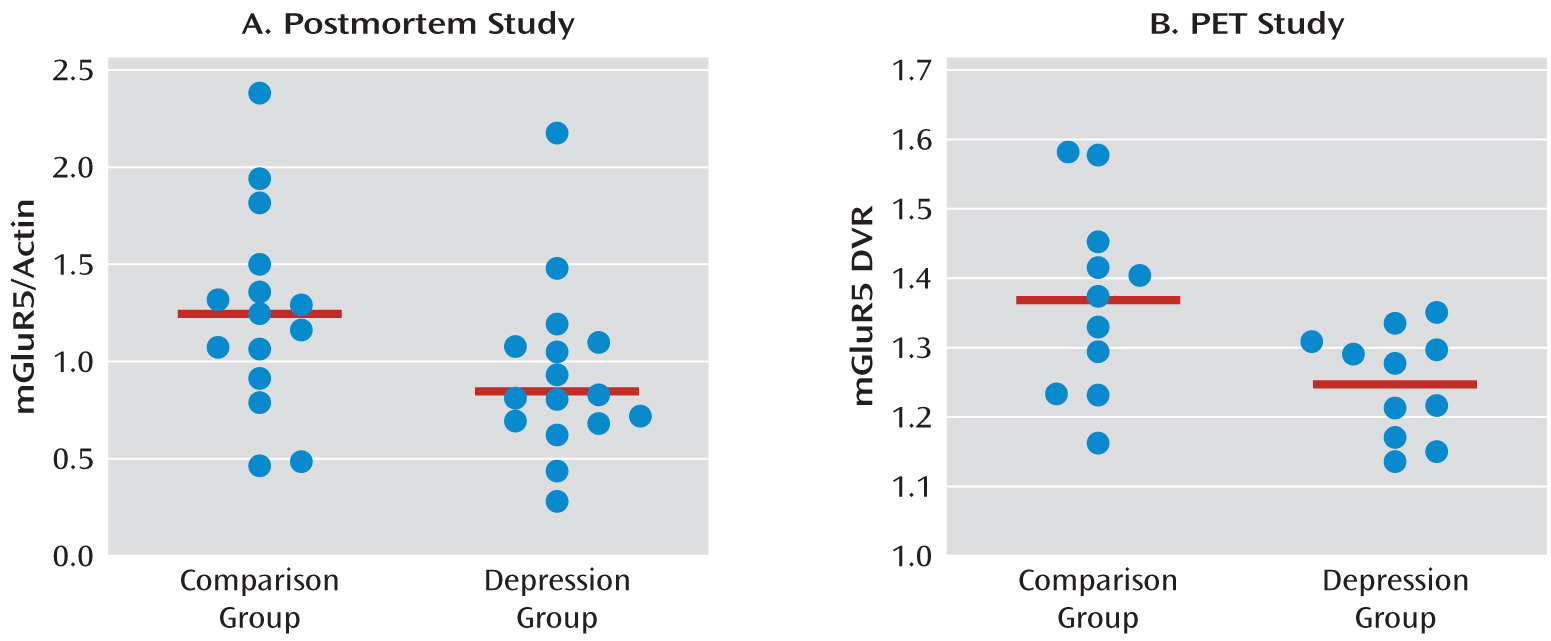
This is the first study to analyze mGluR5 binding and protein expression in individuals with major depressive disorder and comparison subjects using imaging and postmortem approaches. In the PET study, we found lower mGluR5 binding in depressed subjects relative to comparison subjects in multiple areas of the frontal, temporal, and parietal cortices and in the right thalamus, the insula bilaterally, the left hippocampus, the left posterior cingulate cortex, and the precentral gyrus. In the depression group, the severity of depressive symptoms was negatively correlated with mGluR5 binding in the hippocampus bilaterally, and anxiety symptoms were negatively correlated with mGluR5 binding in the thalamus and orbital frontal cortex bilaterally, the right frontal polar cortex, and the left mid-cingulate cortex. In line with the PET study results, the postmortem examination revealed lower mGluR5 protein levels in the right frontal polar cortex in depressed subjects relative to psychiatrically healthy comparison subjects. The postmortem study also revealed that the lower level of mGluR5 protein was specific given that we did not detect a difference in mGluR1 immunoreactivity between diagnostic groups.
Abnormalities in the glutamate receptor system have previously been observed in postmortem brain tissue from individuals with major depression and suicide victims (
6). We have reported brain region-specific abnormalities in the NMDA receptor complex in depression (
5,
20). These studies coincide with clinical reports demonstrating the potent antidepressant activity of ketamine, an NMDA receptor antagonist (
2). In addition, several studies have demonstrated that selective mGluR5 antagonists have antidepressant-like effects in animals (
21), and mGluR5 knockout mice showed decreased immobility in the forced swim test, which has been interpreted as an antidepressant-like phenotype (
10). It has been postulated that the antidepressant properties of mGluR5 antagonists may involve inhibition of NMDA receptor-mediated neurotransmission and/or induction of brain-derived neurotrophic factor gene expression in the hippocampus (
22).
In our PET study, the lower regional levels of DVR in major depression indicate reduced binding of [11C]ABP688, which may reflect reduced mGluR5 density, a change in the affinity of the binding site, or increased concentration of an unknown endogenous ligand. A reduction in mGluR5 density is consistent with our postmortem study showing lower mGluR5 protein levels in the prefrontal cortex in major depression. Thus, reduced binding to mGluR5 in depression most likely reflects reduced density of functional receptors because of decreased total protein concentration.
At present, we can only speculate about mechanisms that might explain the widespread lower levels of [11C-ABP688 binding to mGluR5 in depression in the parieto-temporo-frontal regions, including the insula and orbitofrontal cortex, in depression. Reduced mGluR5 binding may represent a biological trait associated with an elevated risk of depression, possibly due to genetic factors. Alternatively, the mGluR5 receptor binding may be reduced because receptor expression was down-regulated, possibly through the influence of repeated stress, increased glutamate activity, or hormonal changes (e.g., glucocorticoids) in depression. The negative correlation between mGluR5 in the hippocampus and depression severity and between mGluR5 in the ventrolateral thalamus and anxiety suggests that reduced mGluR5 binding reflects a primary pathogenic marker or an ineffective compensatory change.
Neuroimaging, neuropathological, and lesion studies have provided consistent evidence that neuronal networks involving the medial and orbital prefrontal cortex and related mesiotemporal and striato-pallido-thalamic structures, as well as cortical areas, play a major role in the pathogenesis of depression (
23). Neuroanatomical experiments in monkeys have shown that the orbital cortex is associated with sensory association areas in the inferior temporal cortex and somatosensory areas associated with the insula (
24). Lower mGluR5 binding in this orbital prefrontal network may be related to impairments in the coding of affective characteristics of stimuli in depression (
23). The other extended cortical network associated with depression is connected with the medial prefrontal cortex and includes regions where we also found lower mGluR5 binding: the frontal polar cortex, the posterior cingulate cortex, the hippocampal formation, and mesencephalic structures (
25,
26). Since this “visceromotor” network is involved in visceral reactions to emotional stimuli (
23), reduced mGluR5 binding in this network may be related to emotional dysregulation and vegetative symptoms in depression.
Several limitations of our study merit comment. Our cross-sectional design could not distinguish whether abnormalities in mGluR5 receptor binding reflected a biological vulnerability to depression or were a consequence of the illness (
27). Another possible limitation is the confounding effect of medication. In the PET study, participants had been medication free for at least 4 weeks before scanning; nevertheless, we found an association between a history of antidepressant use and lower mGluR5 binding in the left precentral gyrus, which suggests that drug effects cannot be fully excluded. In the postmortem study, although depressed subjects were medication free at the time of death (based on postmortem toxicology screening), this does not exclude the possibility that antidepressants had a long-term effect on mGluR5 protein expression.
To avoid potentially painful arterial cannulation in participants in the PET study, we used the bolus-infusion technique and normalized the PET images using the cerebellar radioactivity concentration. The use of the cerebellum as a reference region was based on convincing in vivo and in vitro evidence showing that mGluR5 level is extremely low in the cerebellum relative to the brain regions that are thought to be involved in depression (
28). However, some studies using the mGluR5 radioligand [
18F]FPEB have questioned the use of the cerebellum as a reference region because of the relatively high specific cerebellar binding, particularly in rhesus brain tissue (
29,
30) but also, to a weaker extent, in human brain tissue as measured in a single subject using a relatively nonspecific in vitro screen (“no-wash” assay) (
30). In contrast, a recent study demonstrated that quantification of mGluR5 receptor with [
18F]FPEB with noninvasive modeling using the cerebellum as reference region may be feasible (
31). In support of this, in vitro and in vivo studies have suggested negligible binding in the cerebellum when using ABP688 and validated the use of the cerebellum as a reference region (
28,
32). In addition, the postmortem study did not identify mGluR5 protein expression (see Figure S3 in the online data supplement), and we are not aware of any other study showing detectable expression of mGluR5 protein in the cerebellum using Western blotting. Previous studies of cerebellar mRNA expression have demonstrated the presence (
33) and absence (
34) of mGluR5 mRNA or weak mRNA expression exclusively in Bergmann glia (
35). Taken together, all studies on cerebellar mGluR5 concentration using more specific measures than that used in one contradictory single-subject study (
29) found negligible mGluR5 expression in the cerebellum, which suggests that the cerebellum can be used as a reference region.
In conclusion, the data we report here demonstrate lower levels of binding and protein expression of mGluR5 in depressed individuals relative to nondepressed comparison subjects. The findings suggest that neurotransmission at mGluR5 is reduced in depression, possibly as a result of basal or compensatory changes in glutamate system activity. Implications of these findings are that mGluR5 receptor expression might be well suited for use as a biomarker for depression and as a target for novel antidepressant medications. These findings in living subjects, corroborated in postmortem tissue, should encourage studies designed to investigate genetic and environmental influences on mGluR5 and interactions among mGluR5, ionotropic glutamate receptors, and monoaminergic receptor systems in mood and anxiety disorders.