Autism spectrum disorders (ASDs) are defined by three core symptom areas: impairments in social relatedness, deficits in social communication, and restricted, repetitive behaviors and interests. While advances have been made in ASD intervention research, the vast majority of studies have focused on pediatric populations. Of the children with ASDs, 75%–80% continue to meet ASD criteria in adolescence and adulthood (
1–
4), and ASDs in adulthood are characterized by persistent functional deficits in core and associated symptom domains (
5). The growing needs of adults with ASDs are reflected in a 48% increase in prescriptions of psychopharmacological agents from 1993 to 2001 and increasing prescriptions for older individuals with autism (
6). Selective serotonin reuptake inhibitors (SSRIs) were reported to be the fastest growing class of psychopharmacologic agents prescribed, growing by 3.5 times in that 8-year period (
6).
The interest in SSRIs for the treatment of ASDs stems from a hypothesized role for serotonin (5-HT) in the pathophysiology of ASDs and the similarities between repetitive behaviors in ASDs and obsessive-compulsive disorder (OCD), a condition for which SSRIs are a first-line treatment. It has been hypothesized that dysfunction of 5-HT regulation in ASDs occurs during early developmental periods, results in cortical morphogenetic abnormalities and altered 5-HT neurotransmission, and influences symptom domains such as anxiety and rigidity (
7,
8). The most consistent biological finding is elevated platelet 5-HT levels in approximately 30% of individuals with autism (
9). Genetic findings suggest a role for the serotonin-transporter-linked polymorphic region (5-HTTLPR) promoter locus on the 5-HT transporter gene (SLC6A4), responsible for encoding proteins involved in the metabolism and neurotransmission of 5-HT. Some studies of SLC6A4 indicate a significant transmission bias for the 5-HTTLPR locus of the 5-HT transporter (
10), whereas others suggest no association of SLC6A4 with autism (
11). A hypothesized role for 5-HT dysregulation in the pathophysiology of ASDs is appealing because of the broad effects of 5-HT on other neurotransmitters and on a range of human behavior (
12).
To our knowledge, McDougle and colleagues conducted the only double-blind placebo-controlled trial of an SSRI, fluvoxamine, in adults with ASDs (
13). Fluvoxamine was found to reduce repetitive behaviors and associated symptoms (e.g., aggression, anxiety) with minimal side effects, such as sedation. In 45 children with ASDs, our group conducted a 20-week double-blind placebo-controlled randomized crossover study evaluating the safety and efficacy of fluoxetine (
14). The outcome measures included the Children's Yale-Brown Obsessive Compulsive Scale and a global measure of autism severity. A low initial dose of 2.5 mg/day was used for the first week, followed by a flexible titration schedule based on weight and tolerability. The mean final dose was 9.9 mg/day (SD=4.4), with a mean maximum dose of 10.6 mg/day (SD=3.7). There was significantly greater improvement in the score on the Children's Yale-Brown Obsessive Compulsive Scale with fluoxetine than with placebo and a nearly significant advantage of fluoxetine on the composite measure of global autism severity. Side effects, including insomnia and agitation, did not differ between the fluoxetine and placebo arms.
However, a large-scale multisite trial of citalopram (
15) funded by the National Institutes of Health Studies to Advance Autism Research and Treatment (STAART) network indicated no significant differences between citalopram and placebo in the reduction of repetitive behaviors. The 12-week trial was conducted with 149 children 5–17 years of age with ASDs, and it used a fixed starting dose of 2.5 mg/day and slow titration to the maximum dose of 20 mg/day, depending on weight, tolerability, and response. No differences were found in a variety of efficacy measures, including the children's Yale-Brown scale and Clinical Global Impression (CGI) ratings. However, citalopram was superior to placebo in reducing irritability as measured with the Aberrant Behavior Checklist irritability subscale. Secondary analyses suggested that stratifying for high baseline irritability decreased placebo response, thereby increasing the differences between drug and placebo (B.H. King, unpublished observations). Significantly more adverse events were reported in the citalopram group than the placebo group, and common adverse events in the citalopram group included increased energy, impulsivity, decreased concentration, hyperactivity, stereotypy, diarrhea, insomnia, and dry skin. One serious adverse event was recorded during treatment with citalopram.
Method
Subjects
Subjects between the ages of 18 and 60 years who met the DSM-IV criteria for an ASD were recruited for this study through clinical evaluation by psychiatrists and neurologists with substantial experience with adult ASDs. Standardized measures were administered by reliable research raters and were used to support the diagnosis of an ASD. These instruments were the Autism Diagnostic Observation Schedule–Generic (
16) and the Autism Diagnostic Interview–Revised (
17), which was administered when parents were available to provide developmental history. Given the relatively high-functioning study group, some individuals who met only the social interaction criteria on the Autism Diagnostic Observation Schedule but whose results on the Autism Diagnostic Interview were consistent with a DSM-IV diagnosis were included in the study. Additional inclusion criteria included global severity ratings in the moderate or greater range (CGI score of 4 or higher) and medication-free status. There was no inclusion severity cutoff score on the Yale-Brown Obsessive Compulsive Scale (
18). Subjects with a history of hypersensitivity or side effects while receiving fluoxetine treatment or abnormal ECG, laboratory test, or physical examination findings were excluded from participation. Subjects with schizophrenia, schizoaffective disorder, bipolar disorder, active seizure disorder, or significant hematopoietic or cardiovascular disease were excluded.
Study Design
A diagram of the patient flow appears in the data supplement accompanying the online version of this article. In a double-blind placebo-controlled treatment design, 22 subjects were randomly assigned to fluoxetine and 15 to placebo. The unequal numbers of subjects in the two treatment arms were the result of uncompleted block randomization. Study medications were dispensed in identical double-blind fashion with a fixed schedule for the first week, starting with one 10-mg capsule in the morning after breakfast to minimize gastrointestinal side effects and insomnia. In the second week the dose was increased by 10 mg, and it was increased by 20 mg each subsequent week as tolerated by the patient, up to a maximum dose of 80 mg/day. The minimum dose for continuation in the study was 20 mg/day.
Efficacy ratings were collected biweekly by two types of masked raters: 1) treating clinicians, psychiatrists or neurologists, who adjusted the medication dose, measured adverse events, and monitored efficacy, and 2) independent evaluators, doctoral- and master's-level psychologists who monitored efficacy while blind to adverse events and dosing schedule. Both types of raters collected CGI improvement ratings for global symptoms and for obsessive-compulsive symptoms. Only the independent evaluators administered the Yale-Brown scale, as in most clinical trials.
Measurement of Repetitive Behaviors
The compulsion subscale of the Yale-Brown Obsessive Compulsive Scale (
18) is a 10-item clinician-rated questionnaire with a 5-point scale rating time spent, distress, interference, resistance, and control of compulsions. This assessed three domains of repetitive behaviors: higher-order (e.g., compulsions), lower-order (e.g., stereotypies), and hoarding. The obsession subscale of the Yale-Brown scale was not used, as obsessions are difficult to assess in this population.
The CGI improvement rating for repetitive behaviors encompassed both repetitive behaviors measured by the Yale-Brown compulsion scale and obsessive thought patterns observed in higher-functioning adults with ASDs. These ratings were based on all available information, including rating scales, clinical observations, and patient report.
Other Outcome Measures
A global rating was obtained with the CGI improvement rating of overall symptoms. This reflected global changes in core and associated symptoms of ASDs. The ratings were based on all available information, including rating scales for repetitive behaviors, depression, and anxiety; clinical observations of core and associated symptoms; and patient report of overall functioning.
The irritability subscale of the Aberrant Behavior Checklist (
19) was used to assess emotional and behavioral symptoms of irritability, e.g., aggression toward others, deliberate self-injuriousness, temper tantrums, and quick changes in mood.
The 17-item Hamilton Depression Rating Scale (HAM-D) was also administered, to assess patterns and severity of depression.
Safety Assessments and Schedule
Safety assessments by the treating clinicians included open-ended questioning at baseline and at subsequent weekly visits. The behavioral and emotional outcome assessments were conducted every 2 weeks. Suicidal ideation was assessed monthly with the suicide item of the HAM-D. Blood samples for measurement of serum levels of fluoxetine and norfluoxetine were drawn at the baseline and endpoint visits to document drug levels and evaluate compliance.
Statistical Analysis
Mixed-effects regression with random slopes and intercepts using an unstructured error covariance matrix with observations nested within subjects were used to test the hypothesis that fluoxetine treatment would result in significant reductions in repetitive behaviors as measured with the Yale-Brown Obsessive Compulsive Scale. A similar model was used to assess change on the irritability subscale of the Aberrant Behavior Checklist. Testing of the CGI improvement ratings of obsessive-compulsive and global symptoms used nonparametric analysis (risk ratio, Fisher's exact test) of differences in response rates between the fluoxetine and placebo conditions. For subjects with missing data at endpoint, the week 12 CGI improvement rating was derived by using maximum likelihood estimation. Responders were defined as subjects with a CGI improvement rating of 1 (very much improved) or 2 (much improved) at endpoint.
Discussion
To our knowledge, the present study is the first randomized placebo-controlled study of the safety and efficacy of fluoxetine in adults with ASDs. The most robust findings indicate significant improvement in the core symptom domain of repetitive behaviors. The repetitive behavior domain has been historically understudied, has been shown to be the most persistent domain across development (
21), is highly heritable (
22), and causes considerable impairment in the daily lives of individuals with ASDs. Since our study did not use high severity of repetitive behaviors as an entry criterion, these results may be generalized to patients across all severity levels of repetitive behavior. The present study also found greater improvements in overall severity and autistic symptoms in the fluoxetine group than in the placebo group.
Our study used the compulsion subscale of the Yale-Brown Obsessive Compulsive Scale, which included three domains of repetitive behaviors: higher-order repetitive behaviors (e.g., compulsions), lower-order repetitive behaviors (e.g., stereotypies), and hoarding. The baseline target compulsive symptoms most commonly reported in patients were repeating rituals (e.g., rereading or rewriting); ordering and arranging; a need to tell, ask, or confess; a compulsion to do things until they are “just right”; adherence to routines and schedules; rigidity; and finger picking or nail biting. Future studies should track patients' chief complaints to allow for a more comprehensive understanding of which specific repetitive behaviors are improving.
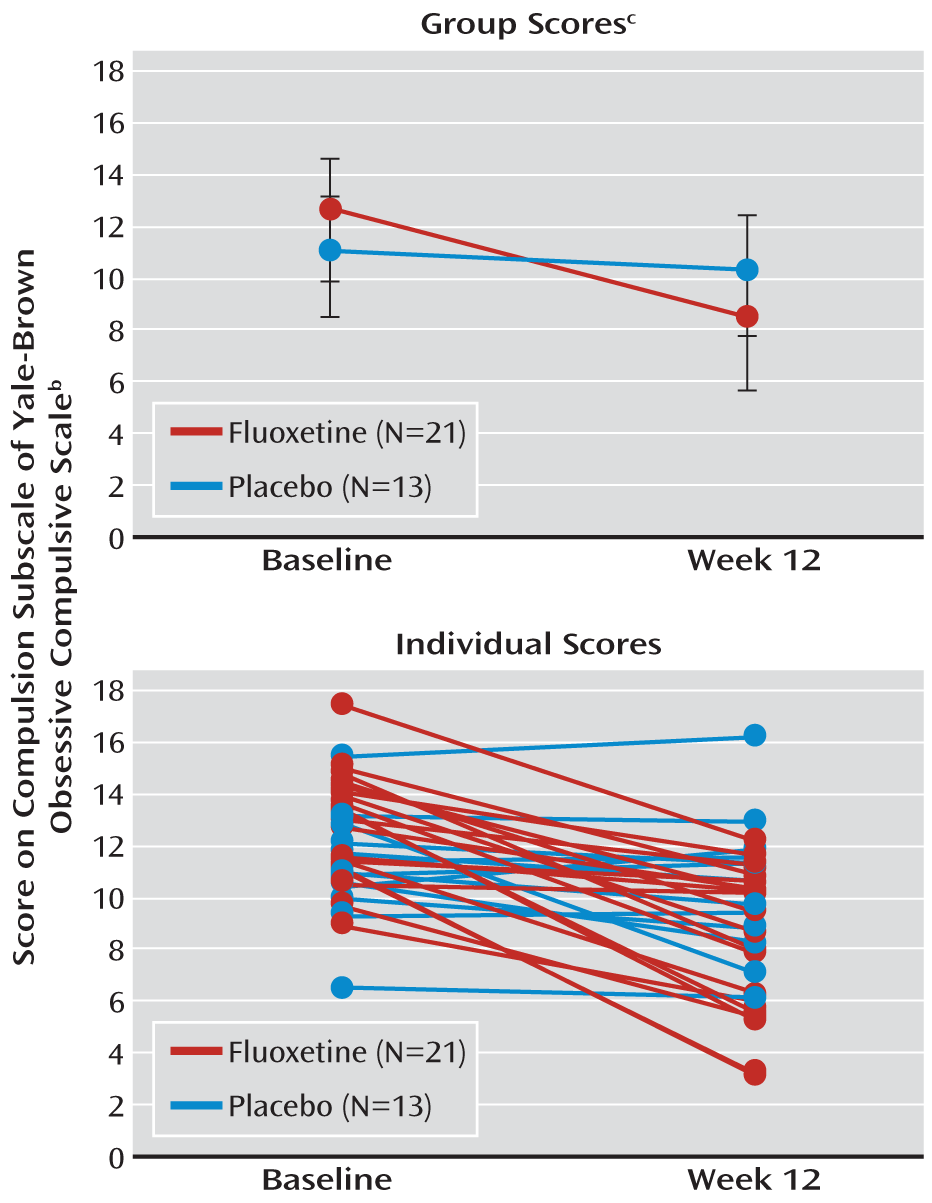
Results from our primary analysis demonstrated clear differences in the slopes for the fluoxetine and placebo groups. The individual subject slopes further support the difference between treatment groups (
Figure 1). Of note, the mean compulsion score on the Yale-Brown scale for the fluoxetine group was numerically higher than the score for the placebo group at baseline but numerically lower than the placebo group's score at endpoint. This may suggest that fluoxetine has a more favorable effect on individuals with a high compulsion level at baseline, but it requires further study.
The CGI rating of improvement in obsessive-compulsive symptoms, which encompasses both the repetitive behaviors measured by the Yale-Brown compulsion subscale and obsessive thought patterns (derived from rating scales, clinical observations, and patient report), also showed significantly greater improvement with fluoxetine than with placebo. However, only the differences in the ratings by the treating clinicians reached statistical significance; those by the independent evaluators did not. In contrast, there was significant improvement in scores for compulsive behaviors on the Yale-Brown scale, which was rated by the independent evaluators. This discrepancy may reflect the different sources of information used by the two groups of raters; the ratings of the treating clinicians were based on broader evaluations of physical, social, and quality of life indicators. Alternatively, it may suggest that the Yale-Brown scale is a more sensitive instrument for assessing compulsions in this population than the CGI improvement rating for obsessive-compulsive symptoms.
Fluoxetine was well tolerated, with only mild to moderate side effects in the treatment group, including insomnia, dry mouth, and headaches. Suicidal ideation was uncommon in both the fluoxetine and placebo groups, and no attempts at suicide were reported. Of interest, we titrated the dose up over 4 weeks to a maximum of 80 mg/day, with good tolerability. The mean dose of fluoxetine was 64.76 mg/day, which produced robust serum levels. In contrast to some pediatric groups reported previously (
15), this study group had little activation. This suggests that fluoxetine can be titrated faster and doses can be higher in adults with ASDs than in children with these disorders.
This study contributes to limited but promising empirical data (
13,
14,
23) supporting the increased use of SSRIs in ASDs (
6). Similar rates of treatment response, 53% versus 0%, were seen in the single published randomized SSRI trial in adults with ASDs of which we are aware, which compared fluvoxamine and placebo (
13). In the current study, 35% of the individuals taking fluoxetine and 0% of those taking placebo improved on overall global functioning, and 50% and 8%, respectively, improved according to the CGI improvement rating of repetitive behaviors. These results are in accord with our finding that liquid fluoxetine had benefits for pediatric ASDs in a previous study (
14), which showed a significant effect on repetitive behaviors and an effect on a composite CGI measure. The present study indicates a more robust response to fluoxetine in adults than in children, although the daily doses of fluoxetine used in the adults (mean, 64.76 mg) were considerably higher than those used in the children (mean, 9.9 mg) on an absolute basis and relative to body weight.
Results of the studies with fluoxetine to date stand in contrast to clinical research with other SSRIs in ASDs, which include a negative multisite trial of citalopram (
15). The discrepancies may reflect differences in study design, since the positive studies of SSRIs now available were derived from single sites or based on within-subjects designs, both of which produce smaller placebo response rates and therefore have a greater ability to detect treatment effects (
24). In comparison to the pediatric STAART-funded citalopram study, the differential findings may also suggest agent-specific effects, since citalopram is more selective with regard to 5-HT transporter blockade than fluoxetine and since fluoxetine has a longer half-life than citalopram. In addition, during the STAART study, citalopram produced dose-limiting effects on tolerability, including one serious adverse event. In contrast, fluoxetine was well tolerated in our study. Also, another complication of comparisons across studies is the difference in study groups. In particular, the subjects in the present study were older (mean age=34.31 years, SD=14.26) than the individuals in the STAART study (mean=9.31 years, SD=3.12), had higher levels of intellectual functioning (IQ≥70: 92% versus 52%) and adaptive skills (mean score on Vineland Adaptive Behavior Composite: 75.43 [SD=24.88] versus 51.07 [SD=17.90]), and had a higher proportion with Asperger's disorder (65% versus 5%).
It is important to note that significant reductions in irritability on the Aberrant Behavior Checklist were reported in the citalopram study, and in the present study treatment with fluoxetine also resulted in a greater decrease in irritability than did placebo, but the difference did not reach statistical significance. Irritability may be associated with repetitive behaviors and significantly contributes to distress in patients and families, and it could be an important outcome measure or could be used to stratify study groups at entry in future studies. Prospective research on developmental differences in SSRI response, examination of differences in SSRI metabolism in adult and pediatric populations, comparison of different SSRI agents, and different trial designs are needed to better understand contrasting findings in the ASD literature, to identify predictors of treatment response, and to understand agent-specific effects on types of repetitive behaviors.
Data on the efficacy of SSRIs in improving repetitive behaviors and possibly global severity in adults with ASDs is promising. Research is needed to better understand variables influencing treatment response, since minimal responses occurred in approximately half of the patients taking fluoxetine in this study group. A pilot study by Buchsbaum et al. (
23) examined potential predictors of response to fluoxetine and found that higher baseline metabolic rates in the medial frontal and anterior cingulate regions, as shown by positron emission tomography, was associated with response to fluoxetine in a small group of adults with ASDs. Pharmacogenetic studies suggest a role for the 5-HT transporter gene in influencing treatment response to fluoxetine, since a higher response rate has been found in children carrying the long allele of the 5-HT transporter gene (
25). Improvement in global severity in a subset of the subjects in the fluoxetine group is of interest, given the poor functional outcomes associated with ASDs (
21). Global improvement could result from direct effects of treatment on core or associated domains (e.g., socialization, anxiety) and/or from secondary effects of treating repetitive behavior symptoms that interfere with functioning. The mechanism of fluoxetine's action on core or associated symptoms is unknown, although a previous report suggested a neurogenerative role for fluoxetine in mature rodents, since chronic administration restored plasticity in the adult visual system and enhanced synaptic plasticity in other brain regions (
26).
Our study is limited by the small number of subjects and by the lack of long-term data with which to evaluate safety and efficacy after 12 weeks. In addition, only the compulsion subscale of the Yale-Brown Obsessive Compulsive Scale was used, given the focus on observable repetitive behaviors. However, our finding of significant improvements on the CGI improvement rating for obsessive-compulsive symptoms, which reflects changes in both repetitive behaviors and obsessive thought patterns, suggests that fluoxetine may also influence obsessive thought patterns in adults with ASDs. In a factor analysis of the entire Yale-Brown checklist (
27), obsessions were identified as a separate and significant factor in the stereotyped, repetitive behavior domain in ASDs. Thus, it might be beneficial if future treatment research evaluates response related to specific repetitive behavior subdomains, including obsessions, higher-order rituals and routines, stereotyped movements and lower-order routines, and hoarding.
We suggest a number of avenues for future investigation. Long-term efficacy data collected from large study groups by means of assessments specifically designed to evaluate domain-specific and functional improvements in adult ASDs are needed. It would also be interesting to have results from research on combined therapies, such as treatment using an SSRI as an augmentative or preparatory lead-in intervention for cognitive-behavior therapy for functional domains such as social and coping skills. Finally, additional work examining developmental differences in SSRI treatment response in ASDs is needed.
Overall, this study yielded findings that differ from the results in a recent high-profile study of citalopram in ASDs (
15). Given the limited available data in this area and the inability to extrapolate from pediatric data, the current findings advance the treatment literature on adults with autism. Nonetheless, identifying the factors underlying these seemingly discrepant findings will be important for developing treatment models for repetitive behaviors in ASDs, for individualizing treatments, and for establishing the best methods for measuring meaningful treatment response.