Interest in the developmental trajectory of bipolar disorder has increased in recent years, in the hope that a developmental perspective will lead to better prevention and treatment (
1). While considerable research on development involves the use of family history or longitudinal designs (
2), few studies utilize neuroscience approaches. In this study, we used functional MRI (fMRI) and the stop-signal task (
3) to map the neural correlates of motor inhibition in pediatric and adult bipolar disorder patients.
Motor inhibition impairments have been observed in both adult and pediatric bipolar disorder. Studies clearly implicate deficient motor inhibition in the adult form of the disorder (
4), whereas data for pediatric bipolar disorder are less definitive (
5). In adult bipolar disorder, motor inhibition deficits (
4) have been linked to impulsivity during mania (
6). In healthy adults, stop cues activate the ventral prefrontal cortex, enabling presupplementary motor area-mediated inhibition (
7) via striatal circuits (
8). Response conflict from these cues is detected by the anterior cingulate cortex (
9). Data comparing the neural correlates of motor inhibition in healthy youths and adults are inconsistent, with some studies reporting increased prefrontal cortex activation in youths (
10,
11) and others reporting the reverse (
8).
Response inhibition in youths with bipolar disorder has been associated with decreased striatal, ventral prefrontal cortex, and anterior cingulate cortex activation compared with healthy youths (
12). However, interpretation of these findings is complicated by comorbid attention deficit hyperactivity disorder (ADHD) in bipolar disorder, since both diagnoses are characterized by impulsivity. Differences in ventral prefrontal cortex and anterior cingulate cortex activation have been reported between youths with manic depression and healthy youths during a task requiring motor inhibition (
13). Finally, during response inhibition, adults with mania exhibit decreased activation in the ventral prefrontal (
14) and anterior cingulate (
15) cortices compared with neural activation in healthy adults.
Overall, this literature generates three conclusions: that motor inhibition deficits exist in bipolar disorder and are related to impulsivity; that motor inhibition is mediated by frontostriatal circuitry that is dysfunctional during inhibition in bipolar disorder; and that the functional circuitry mediating inhibition varies developmentally in healthy individuals. However, to our knowledge, no study has directly contrasted the neural correlates of motor inhibition in adult and pediatric bipolar disorder. Using event-related fMRI, we tested for age group-by-diagnosis interactions during successful and unsuccessful inhibition on the stop-signal task. Patients with ADHD were excluded because comorbid ADHD complicates the interpretation of neuroimaging findings (
12). Available data from the literature suggest three regions of interest: the anterior cingulate cortex, the ventral prefrontal cortex, and the striatum. Based on this literature, we hypothesized that compared with healthy volunteers, bipolar patients would exhibit reduced levels of activity in these regions during failed inhibition (
12–
14) and that given evidence of increasing inhibitory ability throughout adolescence (
16), this effect would be most pronounced in youths.
Method
Participants
Participants were part of an ongoing institutional review board-approved study at the National Institute of Mental Health (Bethesda, Md.). Adults and parents/guardians of children provided informed consent, while children provided informed assent.
Patient recruitment was conducted through advertisements directed at support groups and clinicians. Healthy volunteers (children, N=21; adults, N=29) were recruited from the community through advertisements; they had no lifetime psychiatric diagnoses or first-degree relatives with a mood or anxiety disorder. Exclusion criteria for all participants were an IQ <80, substance abuse within the past 3 months, major medical illnesses, a neurological disorder or neurological damage, comorbid ADHD, and pervasive developmental disorders. No participants were related.
Children were assessed for axis I disorders, including ADHD, with the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (
17). Bipolar children (N=16) met criteria for narrow phenotype bipolar disorder, with at least one hypomanic (≥4 days) or manic (≥7 days) episode with abnormally elevated mood or grandiosity and at least three criterion B mania symptoms (
18). Thirteen children (81.3%) had type I bipolar disorder, and three (18.8%) had type II. Adults were assessed for axis I disorders using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders–Research Version, Patient Edition (
19) or the Diagnostic Interview for Genetic Studies (
20). For all adults (N=23), ADHD was assessed using the ADHD module of the Diagnostic Interview for Genetic Studies. The adult bipolar group consisted of 14 (60.9%) patients with type I bipolar disorder and nine (39.1%) with type II. Interviewers were master's- or doctoral-level clinicians with excellent interrater reliability (kappa values, >0.9 for all diagnoses).
In pediatric bipolar patients, mood was assessed within 48 hours of scanning using the Children's Depression Rating Scale (CDRS) (
21) and the Young Mania Rating Scale (YMRS) (
22). In the adult patient group, mood was assessed within 48 hours of scanning using the Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders Version (SIGH-SAD) (
23) and Young Mania Rating Scale.
Of 153 individuals scanned, data were excluded for 64 (41.8%), specifically for excessive movement (19.6%), poor performance (i.e., <55% accuracy on go trials) (13.1%), equipment failure or poor scan quality (7.2%), and abnormal clinical findings (2.0%). More children than adults (p<0.01) were excluded for movement. The final study sample (N=89) includes 16 children with bipolar disorder and 21 healthy children, for whom data have been previously reported (
12).
Stop-Signal Task
For the stop-signal task (
3), a white cross appeared at the center of the screen for 500 msec at the start of each trial. The cross was replaced by an “X” or “O” go signal for 1000 msec. Using a button-press response box, participants were instructed to press “1” for an “X” presentation and “2” for an “O” presentation. Participants were instructed to respond within 1000 msec unless the stop signal appeared (i.e., the background color changed to red). This occurred on 25% of trials (i.e., stop trials). Participants were to refrain from pressing the button if the stop signal appeared.
On the first stop trial, the stop signal appeared 250 msec after the go signal. Subsequent stop-signal timing was adjusted trial-by-trial based on participant performance. When a participant inhibited successfully, the next stop signal appeared 50 msec later than it appeared on the previous stop trial; otherwise, the signal appeared 50 msec earlier. Trials were separated by 750 msec.
Before scanning, patients and comparison subjects were trained to a mean reaction time <1000 msec on go trials. During scanning, participants completed four runs, each consisting of randomly presented go (N=32), stop (N=16 [eight correct and eight incorrect]), and fixation (N=16) trials. This resulted in 32 correct and 32 incorrect stop trials per participant.
Scanning Acquisition
Scanning was conducted using a General Electric Signal 3T scanner (General Electric, Milwaukee). Participants viewed stimuli through Avotec Silent Vision glasses (Avotec, Inc., Stuart, Fla.). A high-resolution T1-weighted anatomical image following standardized magnetization-prepared gradient echo sequence was collected (180 1-mm sagittal slices; field of view=256 mm; number of excitations=1; TR=11.4 msec; TE=4.4 msec; matrix=256×256; TI=300 msec; bandwidth=130 Hz/pixel, 33 kHz/256 pixels). Gradient echo planar images (23 contiguous 5-mm axial slices/brain volume; parallel to the anterior and posterior commissure line; single-shot gradient echo T2*-weighting [matrix=64×64; TR=2000 msec; TE=40 msec; field of view=240 mm; voxel size=3.75 mm × 3.75 mm × 5 mm]) were collected following manual shim and sagittal localization.
Analyses
Behavioral data.
For each group, we computed the means for accuracy on go and stop trials, for response time on go trials, and for the time between the go and stop-signal onsets (or “inhibit delay”) on stop trials. The stop-signal reaction time was calculated by subtracting the mean go-trial response time from the mean inhibit delay at the point when the participant's accuracy on stop trials was 50% (
3) (if 50% accuracy was not achieved, the mean inhibit delay was subtracted from the reaction time at the
nth percentile of go trials, in which
n was the participant's stop-trial accuracy).
Differences in the means for stop and go accuracy, reaction time, inhibit delay, and stop-signal reaction time were tested independently using separate 2×2 (age group [pediatric, adult]-by-diagnosis [bipolar disorder, healthy comparison]) factorial univariate analyses of variance (ANOVAs). Post hoc contrasts between groups were conducted using Tukey's test.
Imaging data.
We used SPM 8.0 (Wellcome Department of Cognitive Neurology, Institute of Neurology, London [
http://www.fil.ion.ucl.ac.uk/spm]) and the MATLAB 7.0 software package (MathWorks, Natick, Mass.) to examine the images. Preprocessing included slice timing correction, motion correction, spatial normalization to Montreal Neurological Institute space, and smoothing (kernel full width at half maximum=8). At the participant level, event-related response amplitudes were estimated using the general linear model. Event types were unsuccessful stop (stop incorrect) trials, go trials (only correct go trials were included), and successful stop (stop correct) trials. Three contrasts were used for the primary analysis: stop correct versus go; stop correct versus stop incorrect; and stop incorrect versus go. These contrasts controlled for response accuracy, task demand, and presence of a motor response, respectively. Individual contrast images were created using pairwise comparisons of event-related response amplitudes and then entered into second-level random-effects group analyses. A high-pass filter (0.0078 Hz) was used.
Motivated by previous findings (
12–
15), we used small-volume-corrected region of interest analyses to test our hypotheses. Since the adult groups differed in mean age, we included age as a covariate of adult data. We used a factorial design to test for a 2×2 (age group-by-diagnosis) interaction in each region of interest (the left and right putamen, caudate, nucleus accumbens, anterior cingulate cortex, and ventral prefrontal cortex) using anatomical templates applied to each participant's data. For each contrast, we 1) identified clusters in which the peak surpassed the p<0.05 threshold (using the small-volume-corrected procedure in SPM); 2) averaged the estimated contrast values across these clusters for each participant; and 3) conducted univariate ANOVAs and Tukey's post hoc tests in SPSS to clarify group activation differences in the clusters. We report the p values from these region-of-interest analyses as well as the Bonferroni-corrected p values that account for the fact that we examined activation in five regions of interest.
To decompose the interactions from the primary contrasts, we compared group differences on stop-incorrect versus fixation trials, stop-correct versus fixation trials, and go versus fixation trials separately and in clusters identified by the primary analysis. In addition, an exploratory whole-brain analysis was conducted for each primary contrast using a statistical threshold of p≤0.005 and a spatial extent of 20 contiguous voxels (
24).
Separate exploratory post hoc analyses examined the effect of mood state, medication status, and comorbid oppositional defiant disorder, since bipolar children and adults differed on these variables. To test whether the primary effects remained significant when controlling for these differences, we stratified the bipolar disorder sample into euthymic and noneuthymic and medicated and nonmedicated subgroups. Similar techniques were used to test the effect of oppositional defiant disorder.
Discussion
Recent reports emphasize the importance of studying neurodevelopment in individuals with bipolar disorder (
1,
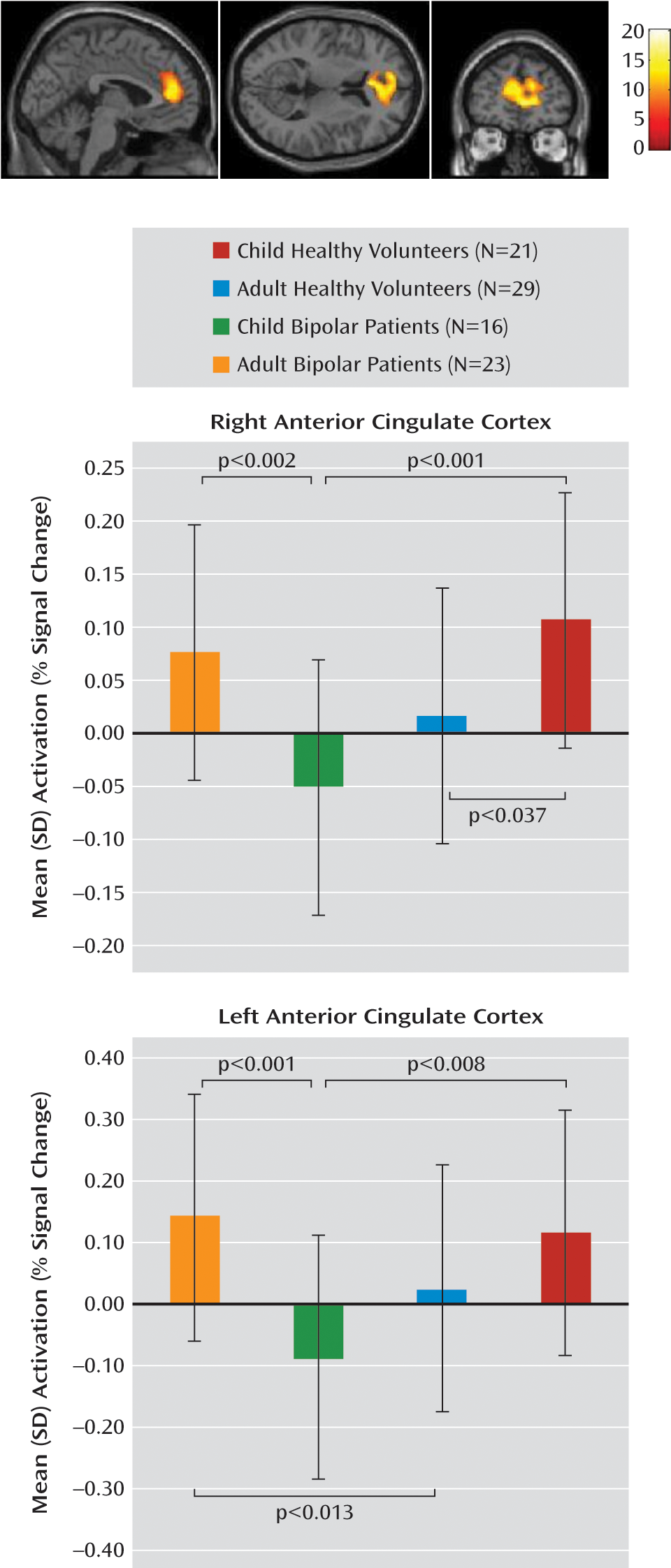
25). Using fMRI during response inhibition in adult and child patients with bipolar disorder and in healthy comparison subjects, we confirmed our hypothesis that bipolar patients without ADHD exhibit frontostriatal dysregulation during unsuccessful response inhibition, and this effect is most pronounced in youths. During unsuccessful inhibition, there was an age group-by-diagnosis interaction, with bipolar youths showing reduced activity in the left and right anterior cingulate cortex relative to both healthy children of similar age and adult bipolar patients, and adult bipolar patients showing increased activation in the left anterior cingulate cortex relative to healthy adults. During successful inhibition, there was a main effect of diagnosis, with healthy subjects showing greater activity in the left ventral prefrontal cortex and the right nucleus accumbens compared with bipolar patients. Dysregulation in the ventral prefrontal cortex, nucleus accumbens, and anterior cingulate cortex during response inhibition exists across development in bipolar patients, suggesting that this dysfunction is central to the pathophysiology of bipolar disorder. Further analyses suggested that our findings were not a result of differences between pediatric and adult patients on mood, comorbid oppositional defiant disorder, or medication status.
The anterior cingulate cortex mediates conflict monitoring and error detection during choice tasks (
9). In our study, healthy youths showed increased anterior cingulate cortex activation relative to healthy adults when making errors, which is consistent with research indicating that error detection develops from childhood into adulthood (
26). Few investigations of bipolar disorder have focused on anterior cingulate cortex activation during motor inhibition, and none have directly compared adult and pediatric patients. In a study comparing motor inhibition in children with mania relative to healthy comparison youths, decreased anterior cingulate cortex activity was observed in the mania group (
13), while in a previous study, adults with bipolar disorder did not differ from healthy comparison subjects (
27). In the present study, pediatric bipolar patients had decreased anterior cingulate cortex activation during failed motor inhibition compared with healthy children and bipolar adults, whereas adult bipolar patients had increased anterior cingulate cortex activation relative to healthy adults. This indicates that error detection during motor inhibition may develop abnormally in individuals with bipolar disorder relative to healthy individuals. Our data support existing evidence that cortical dysfunction is already present in children with bipolar disorder and does not first emerge in adulthood (
13,
24). The anterior cingulate cortex hypoactivation that we observed in the pediatric bipolar group during unsuccessful inhibition is similar to that previously found in bipolar youths during response inhibition, when unsuccessful and successful inhibition trials were analyzed together (
13). Additionally, the anterior cingulate cortex hyperactivation that we observed in bipolar adults adds to existing evidence of anterior cingulate dysregulation during response inhibition, with adult bipolar patients previously showing hypoactivation in this region during successful response inhibition compared with nontargets (
15).
Here, the anterior cingulate cortex was not engaged appropriately in bipolar youths during cognitive conflict, which could diminish the ability of these youths to inhibit inappropriate responses to external cues in real-world environments, such as when they interact with teachers, parents, and friends. Additionally, bipolar adults exhibited anterior cingulate cortex hyperactivation during response conflict, relative to healthy adults, to produce comparable performance. The hyperactivation in bipolar adults may result from functional compensation for the hypoactivation that is present and impairing in childhood. This compensatory hyperactivation in individuals with bipolar disorder may emerge as the anterior cingulate cortex continues to develop throughout adolescence and adulthood (
28,
29). Alternatively, pediatric-onset bipolar disorder may differ from the adult-onset form in certain pathophysiological and clinical features, including anterior cingulate cortex hypoactivation during unsuccessful motor inhibition, mode of inheritance (
30), and increased heritability (
31). These possibilities can be dissociated only by longitudinal studies.
Compared with healthy youths, decreased activation in the striatum, ventral prefrontal cortex, and anterior cingulate cortex during unsuccessful inhibition has been reported in bipolar youths with co-occurring ADHD (
12), while decreased activation in the ventral prefrontal cortex (
14) and anterior cingulate cortex (
15) during response inhibition has been observed in bipolar adults. These previous findings, as well as findings from the present analysis of bipolar youths without ADHD, resemble results from some studies of ADHD. Specifically, anterior cingulate cortex hypoactivation has been reported in youths with ADHD during unsuccessful inhibition (
32,
33), while ventral prefrontal cortex hypoactivation has been reported in adults with ADHD during successful inhibition (
34). This suggests that similar neural dysfunction may be present across diagnoses in patients with shared motor inhibition deficits.
During successful inhibition, healthy volunteers, relative to bipolar patients, exhibited increased activation in the left ventral prefrontal cortex and the right nucleus accumbens. The ventral prefrontal cortex supports inhibition by suppressing basal ganglia output (
35), while the nucleus accumbens receives input from the brainstem important in conditioned learning (
36). Our findings are consistent with studies showing abnormalities in these regions, particularly in bipolar adults (
24,
37,
38). The finding of ventral prefrontal cortex and nucleus accumbens dysfunction in bipolar patients across the developmental spectrum suggests that impaired inhibitory signals to the basal ganglia and aberrant associative learning mechanisms arise early in the illness and persist throughout its course. Longitudinal studies are necessary to confirm this hypothesis.
Our behavioral analysis revealed a main effect of age on go-trial response time and stop accuracy. Compared with adults, the pediatric groups showed faster responses on go trials and decreased accuracy during motor inhibition. Thus, youths use a strategy that sacrifices accuracy for speed, while adults sacrifice speed for accuracy. The age-by-diagnosis anterior cingulate cortex findings in bipolar patients may reflect patient versus comparison subject differences when youths and adults employ these different strategies. Specifically, bipolar youths differ from healthy youths in that hypoactivation occurs in the former in the anterior cingulate cortex during a “speed-driven” strategy, while bipolar adults differ from healthy adults in that hyperactivation occurs in the former in the anterior cingulate cortex during an “accuracy-driven” strategy. Patients and healthy volunteers did not differ on stop-signal reaction time or other behavioral measures, which is consistent with the observation that fMRI measures can be more sensitive than behavioral measures in detecting between-group differences, especially with small sample sizes (
39).
Two important limitations should be noted. We excluded 42% of scanned individuals from our analyses because of excessive movement, poor performance, technical problems, or abnormal brain findings. The exclusion of these individuals, as well as the fact that more children than adults were excluded for excessive movement, potentially limits the generalizability of our findings. However, the percentage of child exclusions is not unusual (
40). Additionally, the effect of medication on imaging results could not be investigated thoroughly, since only one adult bipolar patient was medication free, and ethical considerations complicate the recruitment of medication free patients. However, data (including some for the youths in this study) suggest that medication increases the probability of type II, but not type I, errors (
12).
Cross-sectional comparisons represent a first step in developmental imaging, which ultimately should involve prospective collection of imaging and clinical data. History suggests that cross-sectional comparisons of pathophysiology among children and adults with similar clinical presentations represent a vital first step in the formation of developmentally informed models. To our knowledge, no prior fMRI study has compared pediatric and adult bipolar patients using any cognitive task. We examined motor inhibition, a psychological construct implicated with some consistency in both pediatric and adult bipolar patients. Hence, our results may initiate a new phase of developmentally informed longitudinal imaging.
Our study provides evidence of developmental differences in frontostriatal activation between bipolar patients and healthy subjects. Understanding how brain activation and behavior progress in bipolar disorder can be addressed only through longitudinal fMRI studies. Such studies would help in the accurate diagnosis of pediatric bipolar disorder, in understanding age-specific deficits in bipolar disorder, and in applying intervention strategies appropriate for developmental stage in the illness.