Both children and adults with bipolar disorder exhibit deficits in face emotion processing and abnormalities in amygdala structure and activity (
1–
3). However, to our knowledge, no study has compared bipolar children and adults in amygdala activity during face emotion processing. Such a comparative study could facilitate developmentally sensitive diagnosis and treatment for bipolar disorder (
4,
5). In this cross-sectional functional MRI (fMRI) study, our goal was to begin to fill this research gap.
Studies have reported behavioral deficits during explicit emotion processing (i.e., face emotion labeling) in children with bipolar disorder (
6,
7), in unaffected children at risk for bipolar disorder (
8), and in adults with bipolar disorder (
1,
9). Neural abnormalities during explicit emotion processing have been observed in both bipolar children and adults (
10). However, the most consistent evidence of neural abnormalities in emotion processing in children and adults with bipolar disorder is that of amygdala hyperactivity during implicit emotion processing. During such implicit tasks, emotional features are reflected in aspects of a stimulus, such as the display of emotion in a face, but task instructions focus on nonemotional features of the face, such as the gender of the person depicting the emotion. For example, using this type of implicit task, amygdala hyperactivity has been observed in bipolar children in response to fearful, angry, and happy faces (
3,
11) and in bipolar adults in response to fearful, happy, and sad faces (
12,
13). Thus, in the present study, we used a gender identification paradigm that involves implicit face emotion processing to test for abnormal amygdala activation in child and adult patients with bipolar disorder.
Evidence suggests that the course of bipolar disorder may be more severe in children than in adults (
5,
14) and that younger patients may have greater impairment in face emotion perception than older patients (
1). This in turn suggests that the neural circuitry mediating emotion information processing may be more impaired in youths than in adults with bipolar disorder. Indeed, recent meta-analyses have reported that decreased amygdala volume is observed more consistently in youths than in adults with the disorder (
15,
16). However, these meta-analyses were based on separate structural studies of either youths or adults alone; studies that include both populations and employ identical procedures are needed. In fact, amygdala function in bipolar disorder has not been examined by applying identical methods to assess both adults and children. Such a study will initiate the process of identifying developmental trajectories of amygdala function in bipolar disorder to be pursued in future longitudinal work.
In this cross-sectional study, we compared amygdala activity in children and adults with bipolar disorder and in healthy comparison subjects during implicit processing of angry, fearful, and neutral facial expressions. We included only three facial expressions to ensure an adequate number of presentations of each stimulus type, thus maximizing statistical power while keeping the task brief enough to be tolerable for children. Fearful expressions were selected because they elicit amygdala hyperactivity consistently in both children and adults with bipolar disorder (
3,
12). Angry and neutral expressions were included as a comparison negative emotion and a control stimulus, respectively. To compare neural activity in bipolar children and adults and comparison subjects of similar age, we conducted an amygdala region-of-interest and a whole-brain analysis.
Method
Participants
Bipolar children (ages 7–18 years) and adults (ages 19–60 years) were recruited through advertisements placed on support group websites and distributed to psychiatrists nationwide. Healthy child and adult volunteers were recruited by advertisement. No participants were biologically related.
The 72 participants included in this study were pediatric patients with bipolar disorder (N=18; age range: 9–18 years), adult patients with bipolar disorder (N=17; age range: 20–58 years), healthy child volunteers (N=15; age range: 12–17 years), and healthy adult volunteers (N=22; age range: 20–56 years). Participants enrolled in an institutional review board-approved protocol at the National Institute of Mental Health. Adult participants provided written informed consent. Parents and pediatric participants provided written informed consent and assent, respectively.
Pediatric patients were assessed with the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL) (
17), which was administered separately to children and parents by clinicians with established interrater reliability (kappa ≥0.9). To evaluate mood state in bipolar children, clinicians administered the Children's Depression Rating Scale (
18) and the Young Mania Rating Scale (
19) within 48 hours of fMRI scanning. Pediatric patients met criteria for narrow phenotype bipolar disorder (
20), with at least one full-duration hypomanic episode characterized by abnormally elevated mood and at least three DSM-IV-TR criterion B symptoms for mania. Adult patients were assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (
21) or the Diagnostic Interview for Genetic Studies (
22) by clinicians with established interrater reliability (kappa ≥0.85). The Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders Version (
23), and Young Mania Rating Scale were used to evaluate mood state in adult patients.
Healthy volunteers were medication free and had no lifetime psychiatric diagnoses and no first-degree relatives with a mood disorder. They were assessed using the same diagnostic interviews as patients.
Inclusion criteria were an IQ >70 (as determined by the Wechsler Abbreviated Scale of Intelligence [
24]) and no history of neurological disorder, pervasive developmental disorder, chronic medical illness, or substance abuse or dependence in the past 3 months. After fMRI scanning, 28 of the 100 scanned participants were excluded as a result of scanner malfunction (N=19), excessive movement (N=1), poor image alignment (N=1), or behavioral accuracy below 65% (N=7).
Behavioral Paradigm
The behavioral paradigm we employed has been used previously (
25,
26). Participants viewed gray-scale images of fearful, angry, and neutral expressions of 10 men and 10 women from the Pictures of Facial Affect series (
27). For fearful and angry faces, in addition to standard (100%) expression intensity, lower (50%) and higher (150%) expression intensities were created by morphing with the neutral expression picture. This was done to enhance ecological validity, since individuals normally encounter different face expression intensity levels (
25). To create neutral faces, neutral and happy faces were morphed to obtain a 25% happy expression, since studies suggest that children (
28) and adults (
29) perceive such expressions as neutral.
Using a two-button response box, participants indicated whether the face presented was male or female. Faces were presented for 2500 msec followed by a 500-msec fixation cross. Each of the four runs included 80 face trials (20 neutral trials and 10 trials of each intensity of fearful and angry faces) and 25 fixation trials. The trial order was randomized within each run.
Image Acquisition
Scanning was conducted using a 1.5-T General Electric scanner (General Electric, Milwaukee). Functional data were acquired using multislice gradient echo-planar sequence (31 axial slices, 4-mm thick, voxel size=3.75 mm × 3.75 mm × 4 mm, TR=3000 msec, TE=30 msec, flip angle=90°, field of view=240 mm, matrix size=64×64). Anatomical T1-weighted three-dimensional spoiled gradient-recalled acquisition steady-state images with inversion recovery prep pulse (128 axial slices, 1.5 mm thick, TR=8.1 msec, TE=3.2 msec, flip angle=20°, field of view=240 mm, matrix size=256×256) were acquired to be coplanar with the fMRI scans for spatial registration.
Behavioral Data
We used SPSS (SPSS, Inc., Chicago) to conduct a three-way repeated-measures analysis of covariance (ANCOVA) with age group (child, adult) and diagnosis (bipolar disorder, healthy volunteer) as between-subject factors and emotion (fearful, angry, neutral) as a within-subject factor to compare accuracy and reaction time. Since there was a nearly significant difference in IQ between groups, IQ was included as a covariate. Post hoc ANCOVAs were performed using SPSS.
fMRI Data
Data preprocessing.
Functional imaging data were preprocessed and analyzed using AFNI (Analysis of Functional NeuroImages [
30]). The first four images of each run were discarded to account for magnetic equilibrium. After slice time correction, images within each run were realigned to the fifth image to correct for movement. Images with motion greater than 2 mm in any direction were censored. If more than 5% of a participant's total images were censored, the participant was excluded. After motion correction, realigned functional images were coregistered to anatomical images, and functional images were anatomically normalized to Talairach space. Images were spatially smoothed with 6-mm root-mean-square deviation Gaussian blur.
At the individual participant level, general linear models were used to estimate the shape of the hemodynamic response to each event type (fearful, angry, and neutral expressions). To account for baseline drift and residual motion artifact, regressors included six motion parameters obtained during coregistration and a third-order baseline drift function. Regressors were created for each event type. Regressors for fearful and angry expressions were weighted according to emotional intensity (1 for 50%, 2 for 100%, and 3 for 150%). Trials with incorrect answers were analyzed separately by including a regressor that accounted for incorrect behavioral responses. All regressors were convolved with a gamma-variate hemodynamic response function. Beta coefficients from the individual participant level were oriented to the standard Talairach-Tournoux space and then resampled to a resolution of 3 mm3.
Region-of-interest analysis.
Anatomic masks of the right and left amygdala were created based on the Talairach-Tournoux Daemon. The masks were resampled to match their resolution to the fMRIs. Blood-oxygen-level-dependent (BOLD) signal change from each event type versus the fixation was averaged across all amygdala voxels and entered in SPSS for the group-level analysis. An omnibus three-way repeated-measures ANCOVA with age group (child, adult) and diagnosis (bipolar disorder, healthy volunteer) as between-subject factors and emotion (fearful, angry, neutral) as a within-subject factor was performed for the right and left amygdala. Since reaction time differed between groups, it was included as a covariate in addition to IQ. Post hoc univariate ANCOVAs were performed in SPSS to identify differences between groups.
Whole-brain analysis.
A group-level linear mixed-effects model was conducted with the 3dLME program in AFNI to examine between-group differences in response to the face emotions. The model included age group and diagnosis as between-subject factors and emotion as a within-subject factor, with IQ and reaction time as covariates. Using the 3dClustSim program in AFNI (
http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html), Monte Carlo simulation (10,000 iterations, dimensions: 54×64×50 mm, 3×3×3 voxels, 9×9×8-mm smoothness) indicated that an initial voxel-wise threshold of p<0.001 and a minimum cluster size of 22 voxels yielded a corrected p value of 0.05. Post hoc univariate ANCOVAs were performed in SPSS to identify differences between groups and conditions.
Post hoc analyses.
We conducted post hoc exploratory univariate ANCOVAs in SPSS to test potentially confounding effects of mood state, medication treatment, comorbid illnesses, and bipolar disorder subtype on the region-of-interest results (see the data supplement accompanying the online edition of this article). Because these exploratory analyses included relatively small subsets of bipolar patients, we have provided significant results as well as those that fell short of statistical significance (p<0.10).
Discussion
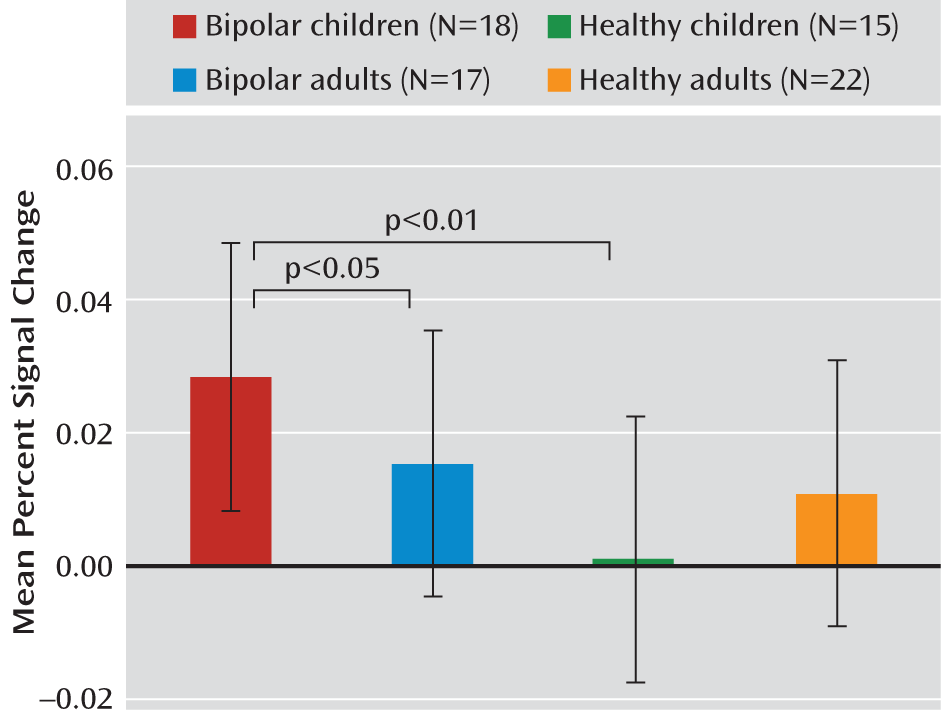
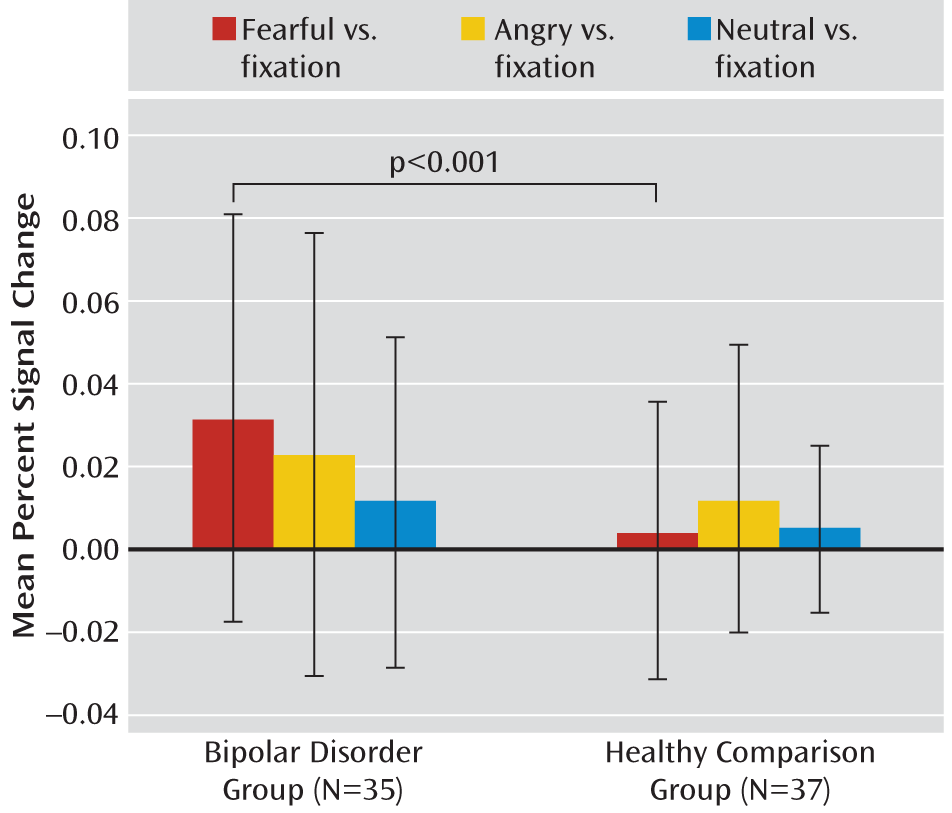
We compared amygdala activity in children and adults with bipolar disorder and healthy volunteers of similar age while they performed an implicit face emotion processing task that included fearful, angry, and neutral faces. Compared with healthy subjects, both children and adults with bipolar disorder exhibited amygdala hyperactivity in response to fearful expressions. However, compared with bipolar adults, children with the disorder exhibited amygdala abnormalities in response to a greater array of facial expressions. The results of this cross-sectional study suggest that there are potential developmental differences in amygdala activity in patients with bipolar disorder. Longitudinal studies are needed to test the hypothesis that amygdala abnormalities persist in response to fearful, but not angry and neutral, expressions as children with bipolar disorder age.
In response to fearful faces, both children and adults with bipolar disorder had increased right amygdala activity relative to healthy volunteers of similar age. This is consistent with results from previous studies that included only children or adults (
3,
10,
12). However, in our study, when amygdala response was considered across all emotions (angry, neutral, and fearful expressions), pediatric bipolar patients exhibited amygdala hyperactivity relative to both bipolar adults and healthy children. Observation of amygdala hyperactivity across expressions in pediatric bipolar patients suggests that there is a more general form of face emotion dysfunction in pediatric bipolar disorder than in adult bipolar disorder. Such an observation is consistent with previous data, including behavioral results. A recent meta-analysis suggested that explicit face emotion processing deficits (i.e., deficits in face emotion labeling across expressions) are observed more consistently in youths than in adults with bipolar disorder (
1). However, it is important to note that abnormalities in implicit and explicit emotion processing may not necessarily be linked (
32). Thus, more fMRI studies are needed to understand the relevance of implicit processing deficits to behavioral explicit processing deficits as well as to examine brain activity during explicit emotion processing in both pediatric and adult patients with bipolar disorder.
Greater cumulative lifetime medication exposure in adult bipolar patients compared with pediatric patients may explain the amygdala activity differences between these two patient groups. In our study, the two groups did not differ on current rates of antidepressant and antipsychotic treatment. Nonetheless, cross-sectional research suggests that such medications may normalize amygdala activity in adults with bipolar disorder (
33). Although to our knowledge this has not yet been tested, it is likely that cumulative exposure to medication may affect amygdala activity. In addition, the developmental differences in amygdala activity that we observed may be associated with differences in the clinical course between adults and youths with bipolar disorder. Although few studies have compared clinical course between the two groups directly, evidence suggests that pediatric bipolar patients may be more likely than adult patients to experience rapid cycling or mixed states (
14). These clinical features, along with early age at onset, tend to be associated with poor longitudinal outcomes (
14,
34). In our study, detailed information about mood cycling or global functioning was not available to test the hypothesis that amygdala hyperactivity across emotions may be linked with worse clinical outcome among pediatric relative to adult bipolar patients. However, this is an important issue for future research.
Our whole-brain analysis revealed that neural activity differences between pediatric and adult patients with bipolar disorder and between bipolar patients and healthy volunteers were not limited to the amygdala. Replicating a previously reported finding, children with bipolar disorder exhibited less activation in the left posterior cingulate cortex than healthy subjects in response to angry expressions (
11). Studies have suggested that the posterior cingulate cortex is involved in top-down control of attention (
35) and in integrating information about emotional expressions, particularly anger (
36). Thus, the abnormal posterior cingulate cortex activity observed in pediatric bipolar patients may be associated with impaired attentional and information processing of angry expressions.
The limitations of this study include its cross-sectional design, since only longitudinal data can support firm conclusions about associations between development and brain activation in individuals with bipolar disorder. First, we cannot disambiguate two relevant hypotheses: 1) that abnormal amygdala activity in children with bipolar disorder becomes specific to fearful expressions as these children age and 2) that throughout a patient's life, pediatric-onset bipolar disorder is associated with more severe amygdala abnormalities than the adult-onset form of the disorder. While children and adults with bipolar disorder differed on some clinical characteristics, our post hoc exploratory analyses suggest that these variables largely did not account for the age-related differences we observed. Second, to ascertain whether differences in amygdala activity between bipolar patients and healthy volunteers represent dysfunction, rather than compensatory mechanisms in response to dysfunction in other brain regions, future studies should examine 1) associations among neural activity during emotional information processing, 2) behavior, and 3) clinical characteristics. For example, if amygdala hyperactivity in patients reflects amygdala dysfunction, one would expect elevated amygdala activity to be associated with behavioral deficits and poor clinical course. In contrast, if such hyperactivation is compensatory to dysfunction in other brain regions, one might expect elevated amygdala activity to predict relatively intact behavior and a relatively benign clinical course. Research examining the compensatory process ideally would follow children prospectively after acquisition of imaging data. In addition, while we were unable to identify aberrant activity in other brain regions to which amygdala hyperactivation might be compensatory, future studies examining larger samples, along with connectivity analyses, could be informative in that regard.
Our study provides the first evidence, to our knowledge, of age-related differences in amygdala activity in response to facial expressions among bipolar patients. Although bipolar disorder patients across age groups showed amygdala hyperactivity in response to fearful faces, the abnormal amygdala activation was present in response to more emotions in pediatric than in adult patients. These findings provide support and guidance for future longitudinal work examining the developmental trajectory of amygdala function, from the asymptomatic risk state through the course of the illness. Knowledge about this trajectory could help in the early detection of bipolar disorder and the development of age-appropriate treatments.