Youths with disruptive behavior disorders, including conduct disorder and oppositional defiant disorder, show increased aggression and antisocial behavior (
1). A subset of these youths also display psychopathic traits, including callous-unemotional (e.g., lack of guilt and empathy), narcissistic (e.g., excessive bragging about one's abilities), and impulsive (e.g., acting without thinking) components (
2). These traits are detectable early in childhood and persist into adulthood (
3). Youths with both disruptive behavior disorders and psychopathic traits are at highest risk for recurrent antisocial behavior (
1,
4). However, functional MRI (fMRI) research investigating the pathophysiology of these disorders and traits has only recently begun (e.g.,
5–
9).
The presence of psychopathic traits, particularly the callous-unemotional component, is thought to reflect emotional processing deficits (
10–
12) and dysfunction in the amygdala (
11,
13), ventromedial frontal cortex, and caudate (
6), although other regions, such as the cingulate cortex, may also be implicated (
14). It is argued that psychopathic traits reflect impairment in stimulus-reinforcement learning and decision making (
13). From this perspective, a reduced amygdala response results in impairment in an individual's ability to learn to avoid actions associated with the distress of others (e.g., a victim's sadness). In line with this, youths with both disruptive behavior disorders and psychopathic traits show reduced autonomic responses to the distress of others (
15), reduced recognition of fearful expressions (
16), and reduced amygdala response to fearful expressions (
7,
8). Moreover, reduced amygdala response to sad expressions in youths with conduct disorder has been reported (
9).
However, an alternative position on psychopathic traits has been prevalent for some time (
17,
18). According to this view, the emotional dysfunction is not primary but rather is a secondary consequence of an irregularity in attention (
17,
18). From this standpoint, the emotional deficits should be understood as a failure to process information that is peripheral to the focus of attention (
18). These problems with deliberate focusing of attention “can be understood as difficulty accommodating bottom-up, stimulus-driven information, especially when the bottom-up information is inconsistent with or unrelated to the current top-down, effortful focus of attention” (
17, p. 346).
Until recently (
19), this attention-based model has been focused on descriptions of information processing functions rather than their neural correlates. However, it is clear that brain regions implicated in top-down attentional control, such as the lateral frontal, dorsomedial, and parietal cortices, affect the amygdala's response to emotional stimuli. Increased priming of task-relevant representations by these regions is thought to reduce the representational strength of emotional stimuli within the temporal cortex, following representational competition (
20), and consequently reduces amygdala responses to these stimuli (
21,
22). In short, the reduced emotional responsiveness of individuals with elevated psychopathic traits might be a secondary consequence of heightened top-down attentional control in response to nonemotional stimulus features (
23).
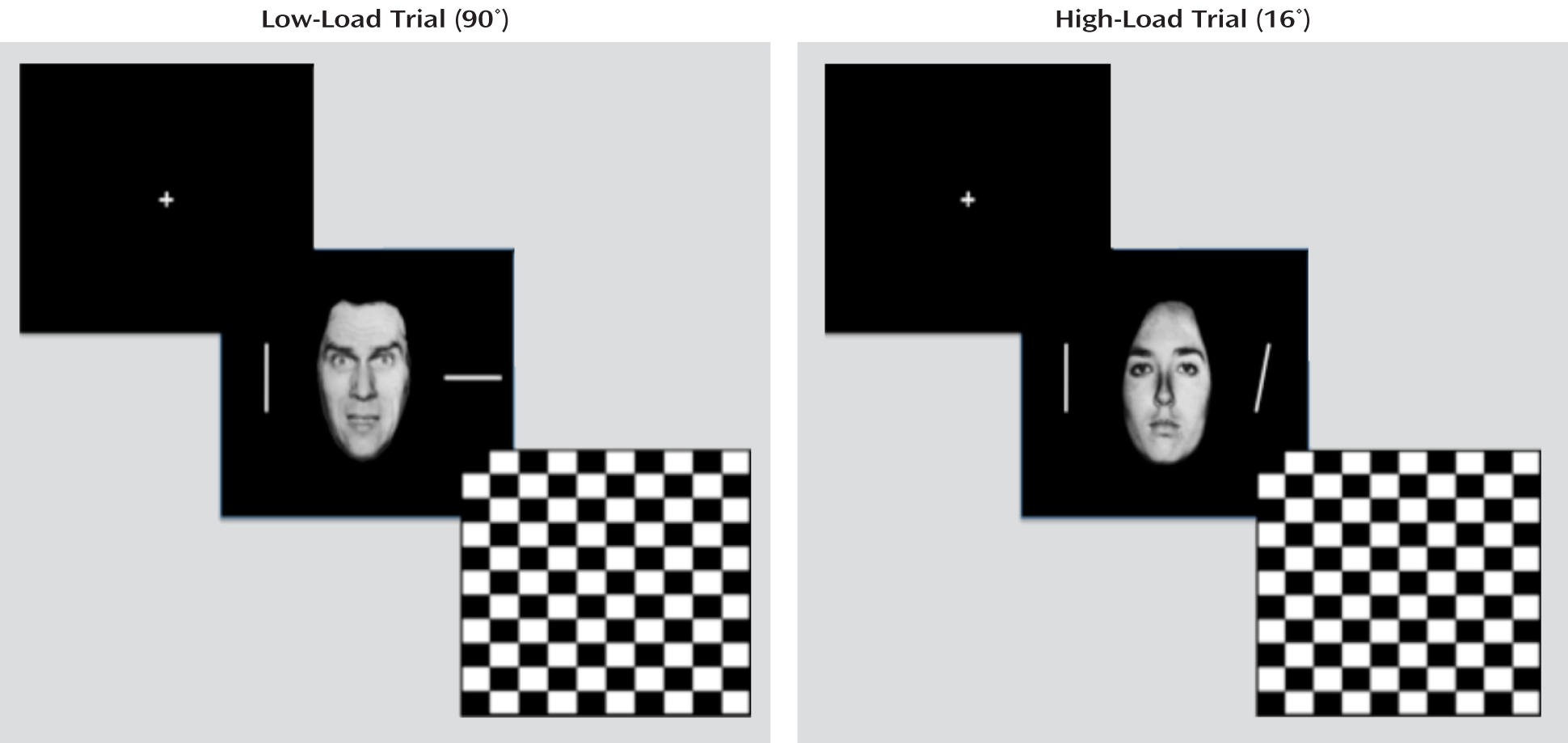
We tested two contrasting hypotheses regarding the basis of emotion dysfunction in youths with disruptive behavior disorders and psychopathic traits. Our first hypothesis was that amygdala-based emotion dysfunction is primary (
11,
13,
14). This predicts that 1) under low attentional load conditions, there will be a reduced amygdala response to fearful expressions in these youths, but under high attentional load conditions, both these youths and healthy comparison subjects will show a reduced amygdala response to fearful expressions, and 2) both groups will show appropriate and equivalent significant increases in activity in brain regions implicated in top-down attentional control (the dorsomedial and lateral frontal cortices) as a function of attentional load. Our secondary hypothesis was that the emotion dysfunction is secondary to attentional irregularities (
17,
18,
24). This also predicts reduced amygdala responsiveness to fearful expressions under low attentional load conditions in youths with behavior disorders and psychopathic traits. However, this position predicts that these youths will show significantly increased responses, relative to healthy youths, within regions implicated in top-down attentional control under low attentional load.
Discussion
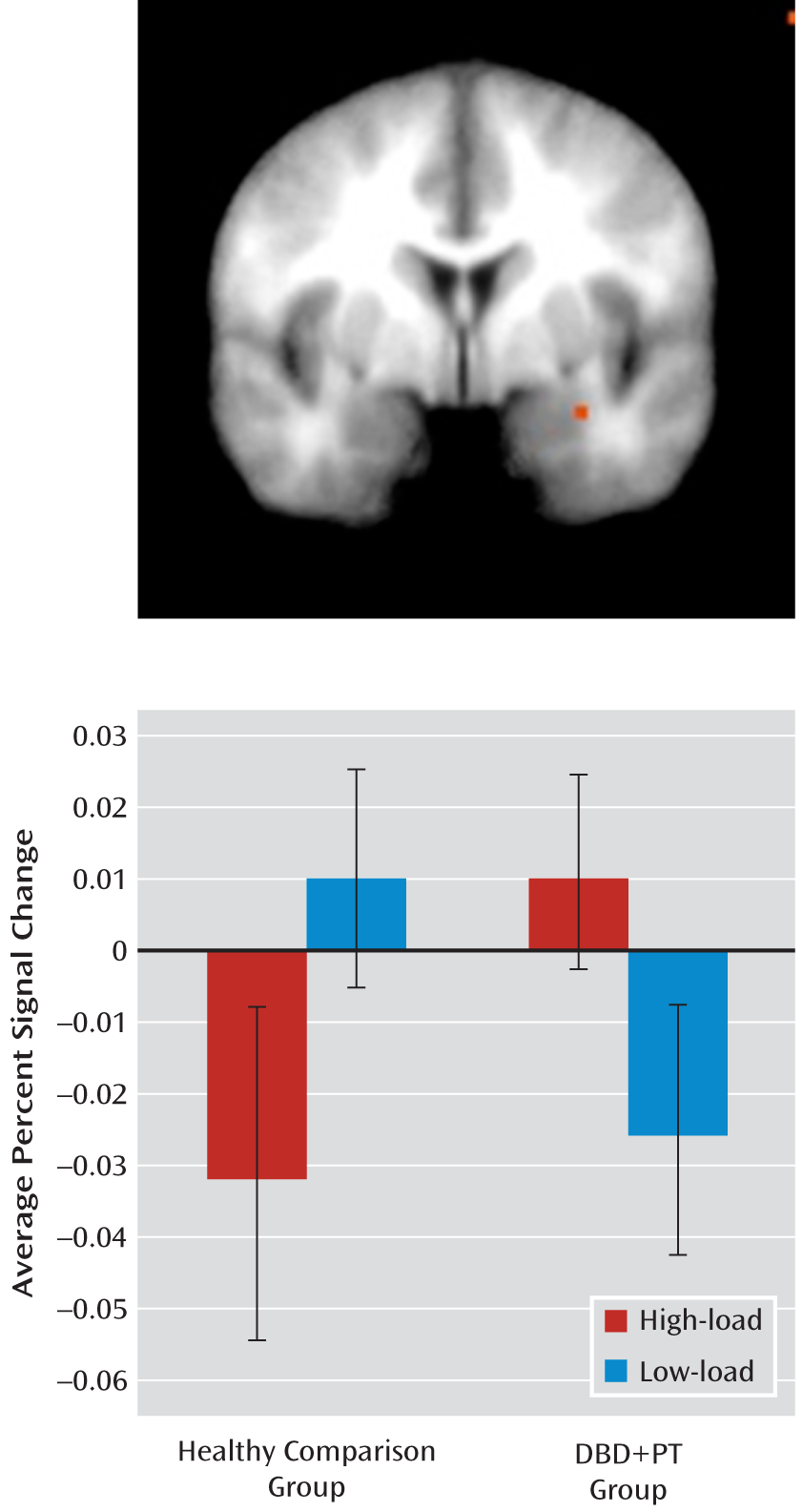
This study examined whether reduced neural response to emotional stimuli in youths with disruptive behavior disorders and psychopathic traits only occurs in the context of heightened recruitment of top-down attentional systems. As expected (
21,
22,
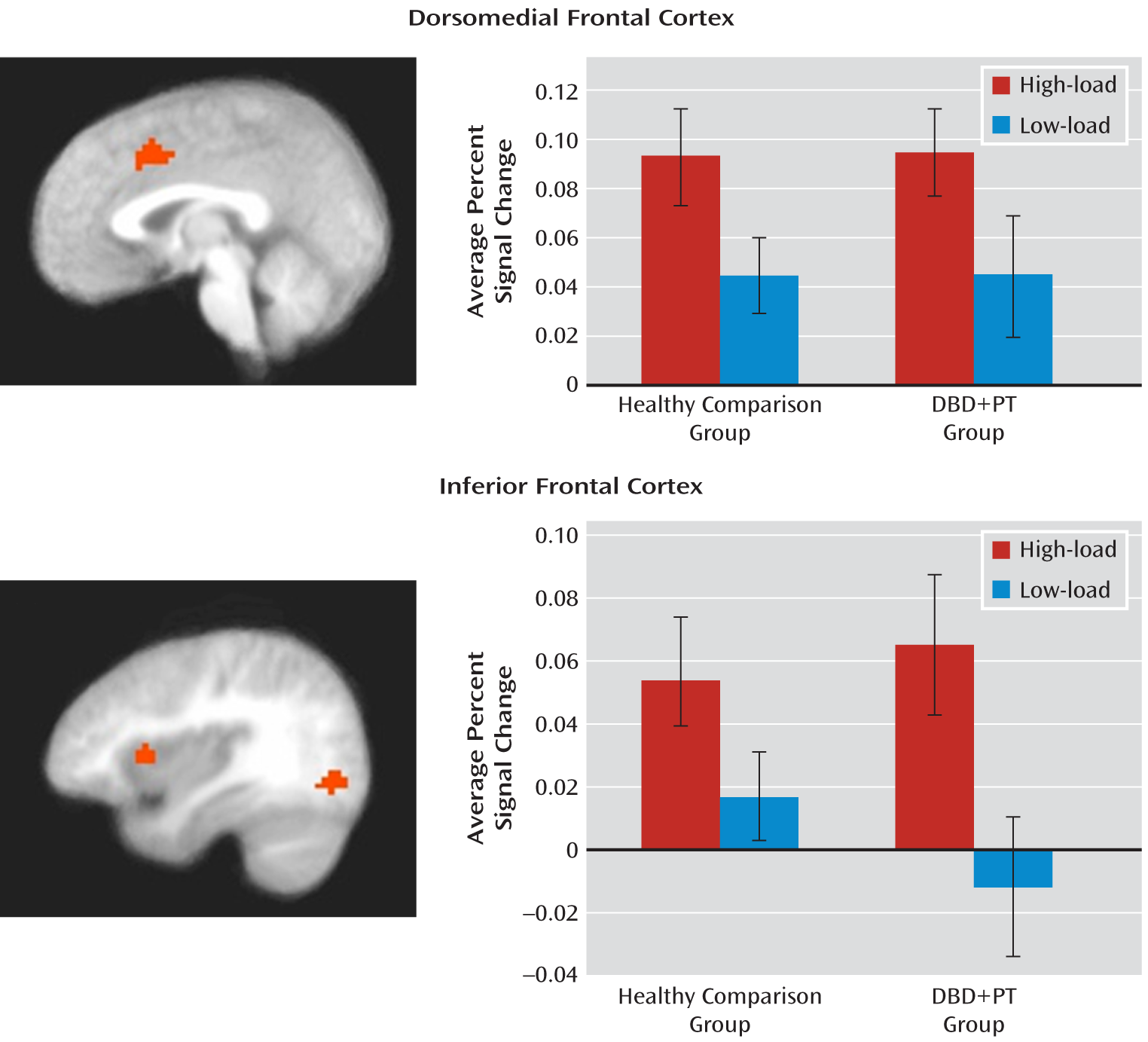
28), healthy comparison youths showed significantly reduced amygdala response to fearful expressions in high relative to low attentional load conditions. In contrast, youths with behavior disorders and psychopathic traits did not. Indeed, the healthy youths showed a significantly greater decrease in amygdala response in high- relative to low-load fear trials compared with youths in the disorders group. Furthermore, symptom severity, as indexed by the callous-unemotional component of psychopathic traits (but not the narcissistic or impulsive component), was found to be significantly correlated with the amygdala response to fearful expressions under low attentional load. Finally, and consistent with previous findings (
21,
22,
28), regions associated with top-down attentional control (the dorsomedial and lateral [inferior] frontal cortices) increased in activation with increased attentional load. Critically, both groups showed equivalent levels of recruitment of these regions with increased attentional load.
Previous studies have reported that youths with disruptive behavior disorders and psychopathic traits show reduced amygdala responses to fearful (
7,
8) and sad (
9) expressions. However, those studies assessed responses to emotional expressions only while participants distinguished the gender of the individual face depicted. From the perspective of attention-based accounts, the previous results could reflect a secondary consequence of heightened processing of the stimulus features relating to gender at the expense of those associated with emotion. Notably, the previous studies did not employ experimental conditions whereby attention was manipulated, and thus they could not systematically address the issue.
Our study echoes previous work (
7,
8) and, critically, demonstrates that youths with behavior disorders and psychopathic traits, compared with healthy youths, show significantly less increase in the amygdala response to fearful expressions under low relative to high attentional load. Moreover, the amygdala response to fearful expressions under low attentional load conditions was negatively associated with symptom severity as indexed by scores on the callous-unemotional subscale of the Antisocial Process Screening Device. According to the attention-based model, this could reflect heightened top-down attentional priming of task-demand stimulus features (the orientation of the bars) and, consequently, reduced representation of emotional features following representational competition (
20) and thus a reduced amygdala response. If this were the case, we would expect to see group differences in the recruitment of regions implicated in top-down attention (the dorsomedial and lateral frontal and parietal cortices) (
20,
34,
35), particularly under low-load conditions. However, this was not observed. Specifically, no increased recruitment of either the dorsomedial or the lateral (inferior) frontal cortex was seen in youths with behavior disorders and psychopathic traits under low-load conditions. In short, our data support the suggestion that the emotional deficit in individuals with behavior disorders and psychopathic traits is primary rather than secondary to increased top-down attention. (The main effect of task load also identified activity in the precentral and lingual gyri. This activity likely reflects increased response planning and selection [precentral gyrus] and increased top-down modulation of posterior visual processing streams [lingual gyrus] [
36].)
It is interesting to note that the inverse relationship between amygdala activity in response to fearful expressions under low attentional load and symptom severity was significant only for the callous-unemotional component of psychopathic traits. Callous-unemotional traits have been previously associated with amygdala dysfunction (
37). Moreover, high callous-unemotional traits have been considered a critical dimension in the development of psychopathy (
38), even though other factors may contribute to heightened scores for the narcissism and impulsivity components of the disorder (
13,
38). Notably, previous research has also particularly associated callous-unemotional traits, as opposed to impulsive antisociality or narcissism, with the primary emotional deficit (
38,
39). In short, our data support the suggestion that amygdala dysfunction causes the compromised emotional responding (callous-unemotional traits) that represents one developmental route to conduct disorder (
13,
38).
There are five caveats that should be considered with respect to the present data. First, although there were high rates of comorbid ADHD within the behavior disorders plus psychopathic traits group, we did not include an ADHD comparison group. This was because previous studies have indicated that youths with ADHD do not present with the pathophysiology found in youths with behavior disorders and psychopathic traits (
5,
8,
40). Indeed, recent work conducted by Posner et al. (
40) found that youths with ADHD showed increased amygdala activation in response to fearful faces. Moreover, and mitigating this limitation, it is important to note that our subsequent group analysis excluding those youths with behavior disorders and psychopathic traits with comorbid ADHD revealed similar results, identifying proximal activations for the interactions and main effects. Second, the medications of four youths with behavior disorders and psychopathic traits could not be withheld at the time of scanning. However, mitigating this limitation, the results of our subsequent ANOVA excluding these participants also identified proximal regions showing significant interactions and main effects. Third, it has been suggested that the aberrant attention proposed to exist in individuals with callous-unemotional traits is a function of time (
19). The suggestion is that individuals with these traits may show typical emotional responses if the emotional stimuli are processed in advance of top-down attentional control. This will be an interesting refinement to examine empirically. However, in our study, the youths with psychopathic traits did not show an appropriate amygdala response to the emotional distracters. According to the attention-based model, this should reflect aberrant attentional control. However, the youths with psychopathic traits showed no evidence of increased recruitment of regions implicated in top-down attentional control that could result in the reduced amygdala responses. Fourth, we did not include a group of youths with behavior disorders without psychopathic traits. Hence, we cannot determine whether our findings are specific to psychopathic traits or to disruptive behavior disorders more generally. However, the significant relationship between amygdala activation and callous-unemotional traits specifically suggests that amygdala responsiveness may mark a developmental route to conduct disorder associated with these traits (
13,
38) rather than to conduct disorder more generally. Fifth, the behavioral performance of youths with behavior disorders and psychopathic traits did not significantly differ from that of youths in the comparison group. Indeed, both groups responded faster to neutral trials relative to fear trials under low but not high attentional load. This suggests that fearful expressions can exert some effect on the behavior of youths with behavior disorders and psychopathic traits even if their neural response to these stimuli is significantly reduced. Of course, the presence of amygdala dysfunction in the behavior disorders plus psychopathic traits group, despite comparable task performance, indicates that group differences in this region stem from neural abnormalities rather than performance differences.
In summary, the present data extend previous findings of amygdala dysfunction in youths with disruptive behavior disorders and psychopathic traits by showing that this emotional deficit is primary and not secondary to aberrant top-down attentional control. Finally, the data suggest that amygdala dysfunction may be particularly associated with the callous-unemotional component of psychopathic traits.