Metformin is the most prescribed insulin sensitizer for the management of type 2 diabetes and may constitute the treatment of choice. Recently, interest in metformin's mechanism of action has been stimulated by the recognition of its pleiotropic actions on several tissues, including skeletal muscles, adipose tissue, endothelium, and the ovary, although the liver is its primary target organ (
19). For example, metformin has been shown to decrease weight in patients with antipsychotic-induced obesity (
15,
16). Metformin appears to affect ovarian function in a dual mode, through the alleviation of the excessive effect of insulin on the ovary and direct effects on ovary (explored in cultures of ovarian cells) (
18).
To our knowledge, there has never been a double-blind placebo-controlled study to assess the efficacy of metformin for antipsychotic-induced amenorrhea and weight gain among female patients with schizophrenia. We report a 6-month randomized, double-blind, placebo-controlled trial that tested the efficacy of metformin in reversing antipsychotic-induced amenorrhea and attenuating antipsychotic-induced weight gain in female patients with first-episode schizophrenia.
Method
Participants
Female patients 18–40 years old with first-episode schizophrenia were recruited from the schizophrenia outpatient clinic of the Mental Health Institute of the Second Xiangya Hospital, Central South University, China, between February 2008 and July 2010. The study was approved by the hospital's ethics committee, and all participants provided written informed consent in accordance with National Health and Medical Research Council guidelines.
The diagnosis of schizophrenia was determined by the Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (
24). To be eligible for the study, patients had to be relatively stable during the screening phase, as indicated by a total score <60 on the Positive and Negative Syndrome Scale (PANSS) (
25); had to have initiated treatment for the first episode of schizophrenia within the past year; had to have experienced amenorrhea within the first year of treatment with one of four antipsychotics—clozapine, olanzapine, risperidone, or sulpiride; had to have been either discharged from inpatient units or first seen in the clinic in the 12 months before enrollment, so their menstrual cycles, weight, and antipsychotic treatment were documented; and had to have taken only one antipsychotic, with no more than a 25% change in dosage, over the past 6 months. Amenorrhea was defined as the absence of menses for 6 months in those who had irregular menstrual patterns or the absence of menses for 3 consecutive months in those who had regular menstrual cycles of 21–45 days. All participants had to be under the care of a parent or another adult caregiver who monitored and recorded medication intake during each day of the trial to monitor adherence.
Patients were excluded from the study if there was evidence of liver or renal dysfunction, cardiovascular disease, or diabetes mellitus; if they were pregnant or lactating; or if they had a psychiatric diagnosis other than schizophrenia or a current history of a substance use disorder.
Study Design
This was a double-blind, randomized, placebo-controlled study. Participants were randomly assigned to receive metformin (1000 mg/day) or placebo for up to 6 months. The dosage of 1000 mg/day of metformin was based on safety and efficacy findings for obese Chinese women with amenorrhea (
20,
21). Randomization was carried out using a computer-generated table to assign patients to one of the two treatment groups in blocks of four to ensure approximately equal numbers of participants in the two groups. To ensure concealment of the randomization, a research pharmacist who was independent of the investigators at a separate facility provided the study medications in coded containers of identical-appearing capsules of metformin or placebo supplied by the manufacturer.
Pharmacological Intervention
For the first 3 days, participants took 250 mg of metformin or placebo twice a day, one before lunch and one before dinner. From day 4 onward, they took 500 mg of metformin or placebo twice a day. Any antipsychotics that patients were taking before enrollment in the study remained at the same dosage throughout the course of the study. Only trihexyphenidyl for extrapyramidal symptoms and lorazepam for insomnia or agitation were considered concomitant medications and were allowed as needed during the study period.
Participants' adherence to the study medication for each visit was defined as having taken more than 80% of the study drug dose prescribed for that period. If a participant was nonadherent, both the patient and the caregiver were counseled on the importance of taking the prescribed study medication.
Measures
The baseline assessment included recording of demographic information, a comprehensive medical history, a physical examination including weight and height measurements, and laboratory tests. The PANSS and the Treatment Emergent Symptom Scale (
26) were used for monitoring psychiatric symptoms and adverse effects. The research nurses who performed all assessments were blind to the treatments. The baseline laboratory tests included fasting glucose and insulin levels; prolactin, luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol, testosterone, and lactic acid levels; liver and renal function tests; blood counts; and ECG. Since fasting blood samples were required, fasting was confirmed with patients or their caregivers when the blood was drawn.
Follow-up visits occurred monthly for 6 months after randomization. At each follow-up visit, all baseline evaluations (including physical examination, amenorrhea state, weight and height measurements, and Treatment Emergent Symptom Scale) were repeated except for the laboratory tests, which were repeated at months 2, 4, and 6. The PANSS was readministered at 6 months.
Glucose and lactic acid levels and liver and renal function tests were determined by enzymatic procedures of the Boehringer Mannheim/Hitachi 714 automated chemistry analyzer. Hormonal levels were determined by using the commercially available Abbott Axsym microparticle enzyme immunoassays (Abbott Laboratories, Abbott Park, Ill.). All of the assays had a within-assay precision of <7% variations and a between-assay precision of <10% variations over the working range. Serum insulin level was measured with a solid-phase radioimmunoassay. Weight and height measurements were made after participants had removed their shoes and upper garments and donned an examination gown.
The primary outcome measures were the proportion of patients whose menstruation was restored, as well as changes in body weight and body mass index (BMI). The major secondary outcome measures were changes in hormone levels, including prolactin, LH, FSH, estradiol, and testosterone; changes in fasting insulin and glucose levels, LH/FSH ratio, insulin resistance index, and PANSS score; and frequency of adverse events. Insulin resistance index was calculated based on the homeostasis model assessment: insulin (10
3 μIU/liter) × fasting glucose (mmol/liter)/22.5 (
27).
Statistical Analysis
All analyses were conducted with SPSS, version 11.5 (SPSS, Inc., Chicago) and SAS, version 9.2 (SAS Institute, Inc., Cary, N.C.), using data from all randomized patients with at least 1 follow-up test (intent-to-treat analysis). The follow-up data (except for the proportion of patients who resumed menstruation) were all repeated measures. The repeated outcome measures were analyzed using mixed-models repeated-measures regression analyses. The subject was treated as the random effect and month as the fixed effect during the mixed-models repeated-measures modeling process. A compound symmetry matrix was specified as the appropriate structure for the variance-covariance matrix used to describe the relationships among the data points.
Because the outcome of restoring menstruation was a dichotomous outcome, binary logistic regression analysis was performed to examine the relationship between changes in body weight, BMI from baseline to month 3, and changes in insulin levels, insulin resistance index, prolactin levels, LH levels, and LH/FSH ratio from baseline to month 4 with restoration of menstruation. A second analysis was conducted with the independent variable changes from baseline to month 6 with restoration of menstruation as the dependent variable. Observed associations were expressed as β coefficients. All these independent factors were entered into the “backward likelihood ratio” binary logistic equation at a single stage with the probability for entry set at 0.10 and removal at 0.15, reporting the β coefficient values that were significant at a two-tailed p level of 0.05.
The sample size calculation was based on the data available on metformin in obese women with amenorrhea (
20,
21). With a total sample size of 84 subjects, 42 subjects per arm, the power of the study was 85%.
Results
Randomization and Patient Characteristics
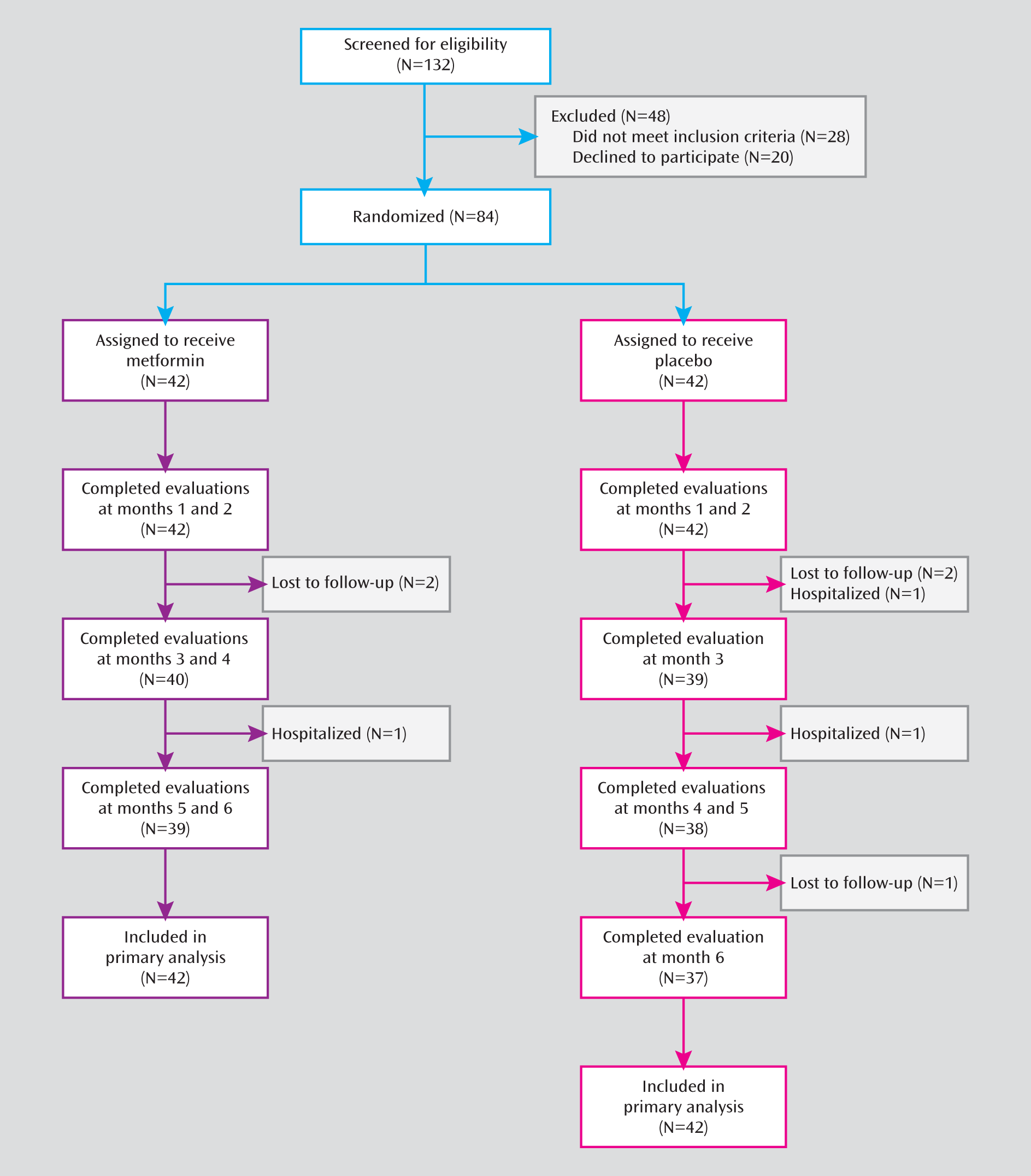
Of the 84 eligible patients, half were randomly assigned to receive metformin, and half to receive placebo (
Figure 1). A total of 76 patients (90.5%) completed the 6-month treatments, 39 (92.8%) of whom were in the metformin group and 37 (88.1%) in the placebo group. The demographic and clinical characteristics and baseline assessments did not differ significantly between the two groups (
Table 1). At baseline, the patients had taken antipsychotic medication for a mean of 6.2 months in the metformin group and 6.5 months in the placebo group. Eight (19.0%) patients in the metformin group and 10 (23.8%) in the placebo group had a BMI above the normal range (reference range: 18.5–23.9). Overall, 59 (70.2%) patients (31 metformin patients and 28 placebo patients) had gained more than 10% of their body weight compared to their weight prior to taking antipsychotics, which is often considered excessive within the first year of treatment. Change in body weight was determined by the weight at the study screening visit and at the first visit (either as an outpatient or an inpatient) to the Mental Health Institute of the Second Xiangya Hospital. As for medication adherence during the study, the patients and their caregivers reported that 88%–100% of patients in both groups took 80% or more of their medications.
Between-Group Difference in Percentage With Restored Menstruation
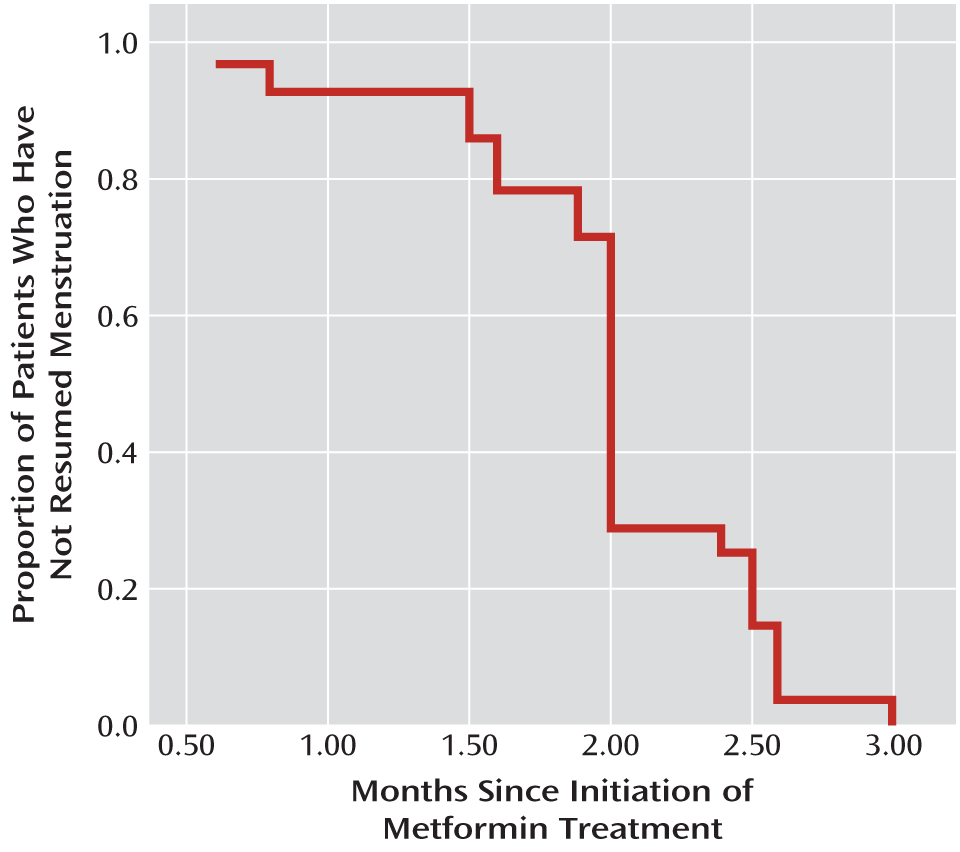
Twenty-eight patients in the metformin group (66.7%) resumed menstruation, compared with only two in the placebo group (4.8%; χ
2=35.05, df=1, p<0.001). As shown in
Figure 2, menstruation was restored in the first 3 months in all 28 patients in the metformin group whose menstruation was restored; the mean time from metformin initiation to menstruation restoration was 2 months (SD=0.5).
Changes in Body Weight and BMI
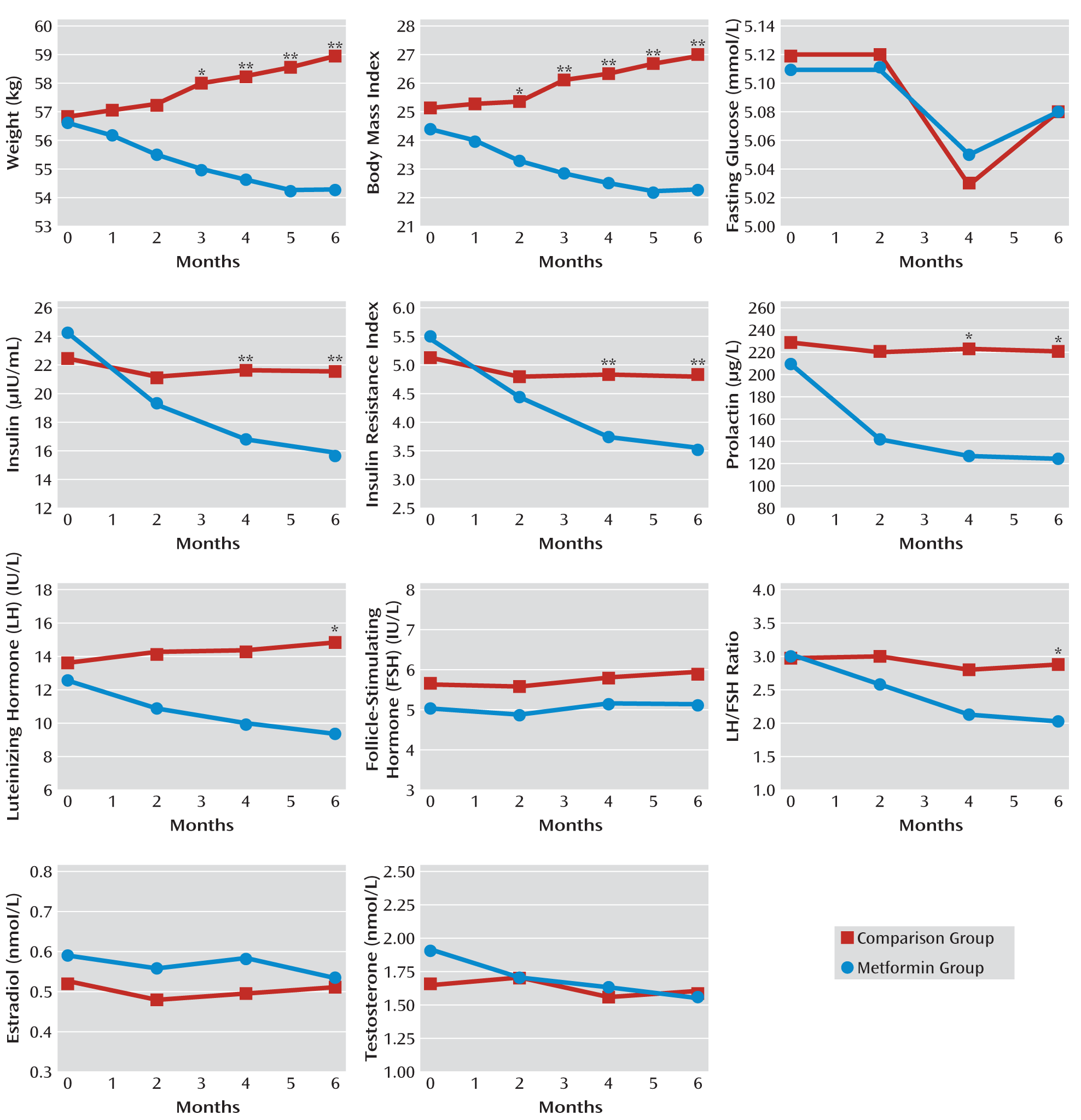
The mean changes in body weight and BMI between the two groups are summarized in
Figure 3 (see also Table S1 in the data supplement that accompanies the online edition of this article). The mean body weight and BMI values decreased significantly in the metformin group at each follow-up session, and increased significantly in the placebo group from month 3 to month 6.
During the 6-month study period, patients in the metformin group lost 4.1% of their body weight from baseline to end of study (mean=2.3 kg), whereas in the placebo group, the mean body weight increased by 3.7% (mean=2.1 kg). Similarly, the mean BMI decreased by 0.93 in the metformin group from baseline to end of study, while it increased by a mean of 0.85 in the placebo group. The decreases in body weight and BMI in the metformin group were significantly greater than those in the placebo group from month 3 to month 6.
Over the 6 months, the mean body weight decreased by 4.0 kg among the 31 metformin patients who had gained more than 10% of their pre-antipsychotic treatment weight and by 1.4 kg among the 11 metformin patients who had gained less than 10% of their pre-antipsychotic treatment weight. However, the mean body weight for patients in the placebo group increased by 2.3 kg for the 28 patients who had gained more than 10% of their pre-antipsychotic treatment weight and by 2.5 kg for the 14 patients who had gained less than 10% of their pre-antipsychotic treatment weight. At 6 months, 12 (28.6%) patients in the metformin group and one (2.4%) in the placebo group had lost >10% of their baseline weight (χ2=11.012, df=1, p=0.001).
Changes in Metabolic Measures
The mean insulin level and insulin resistance index decreased significantly in the metformin group at months 2, 4, and 6 compared with baseline values. In the placebo group, however, there was no significant change in insulin level or insulin resistance index at any of these assessments (
Figure 3; see also Table S1 in the online data supplement). In the metformin group, the mean insulin resistance index decreased by 2.04 from baseline to end of study. The decreases in insulin levels and insulin resistance index in the metformin group were significantly greater than those in the placebo group at months 4 and 6. However, there were no significant changes in fasting glucose levels during the follow-up visits in the two groups.
Changes in Hormone Levels
Significant decreases in mean prolactin level at each follow-up visit were observed in the metformin group but not in the placebo group (
Figure 3; see also Table S2 in the online data supplement). During the 6-month study period, the mean prolactin level decreased by 40.7% (mean=84.87 μg/liter) from baseline in the metformin group. At 4 and 6 months, decreases in prolactin levels for the metformin group were significantly greater relative to those in the placebo group.
The mean LH and testosterone levels and the LH/FSH ratio decreased significantly in the metformin group at 2, 4, and 6 months, whereas there were no significant changes in these measures in the placebo group (
Figure 3).
In the metformin group, over the 6-month study period, the LH level decreased by 26.0% (mean=3.29 IU/liter), the testosterone level by 18.8% (mean=0.36 nmol/liter), and the LH/FSH ratio by 34.1% (mean=1.04) compared with baseline values. The decreases in LH level and LH/FSH ratio in the metformin group were significantly greater than those in the placebo group at month 6. The estradiol level did not significantly change in the two groups during the study period.
Relationship Between Restoration of Menstruation and Changes in Body Weight and Hormone Levels
The mean time to menstruation restoration in the metformin group was 2 months, and all cases occurred in the first 3 months. We performed binary backward logistic regression analysis to evaluate the relationship of menstruation restoration to changes in body weight, BMI from baseline to month 3, and insulin level, insulin resistance index, prolactin level, LH level, and LH/FSH ratio from baseline to month 4. The results showed that the extent of decreases in body weight, insulin resistance index, prolactin level, and LH level contributed to a greater probability of restoration of menstruation, with β values of 1.134 (p<0.001), 0.266 (p=0.002), 0.762 (p=0.011), and 0.546 (p=0.034), respectively.
We used the same binary logistic regression model to evaluate the changes in these variables from baseline to month 6. Decreases in body weight, insulin resistance index, prolactin level, and LH level contributed to a greater probability of restoration of menstruation, with β values of 0.833 (p=0.001), 1.375 (p=0.002), 0.488 (p=0.040), and 0.312 (p=0.033), respectively.
Last-observation-carried-forward analyses, which were conducted separately, revealed findings similar to those of the mixed-models repeated-measures analysis and thus are not presented here.
Adverse Events
There were no significant differences in the frequency and types of adverse events reported between the two groups. Adverse events that affected more than 5% of the overall sample are presented in
Table 2. There were three serious adverse events that led to withdrawal from the trial, one in the metformin group and two in placebo group. All three events were exacerbation of psychosis and resulted in hospital admission. No hypoglycemia was reported during the trial. Serum lactic acid levels, liver and renal function tests, and ECG results remained normal in all patients throughout the course of the study, and there were no episodes of vomiting or lactic acidosis.
Discussion
In this first randomized, double-blind, placebo-controlled, 6-month study of metformin compared with placebo for antipsychotic-induced amenorrhea and weight gain in female patients with first-episode schizophrenia, we found that 66.7% of patients in the metformin group resumed menstruation, whereas only 4.8% in the placebo group did so. Furthermore, the mean body weight, BMI, insulin level, and insulin resistance index significantly decreased from baseline in the metformin group, whereas there were significant increases in body weight and BMI in the placebo group. In our previous studies (
15,
16), we found that metformin could attenuate antipsychotic-induced weight gain and improve insulin resistance. Similarly, Klein et al. (
27) reported that metformin could reduce weight gain in children and adolescents who were treated with olanzapine.
In this study, we also observed statistically significant decreases in mean prolactin, LH, and testosterone levels and LH/FSH ratio in the metformin group. These findings suggest that metformin may improve or reverse antipsychotic-induced amenorrhea and endocrine system dysfunction.
Since metformin has numerous central and peripheral pharmacological effects in humans, the exact mechanism by which it restores menstruation is unknown; alterations in dopamine tone, reduction in weight, decreased prolactin levels, and reduced metabolic syndrome may be potential mechanisms underlying restoration of menstruation (
13,
14). Some studies suggest that antipsychotic-induced menstrual dysfunction is independent of prolactin levels but related to ovarian dysfunction, which can be exacerbated by insulin resistance (
28–
30). Moreover, antipsychotic-induced weight gain may be highly related to abnormal hormone levels, including increased levels of prolactin, estrogen, and testosterone (
31). Prolactin secretion can be modulated by levels of other hormones, such as estrogen (
32), and hyperprolactinemia may be related to multiple factors, including weight gain, metabolic syndrome, and insulin resistance (
33,
34).
Polycystic ovarian syndrome, a common syndrome occurring in 7% of women, can be characterized by hyperandrogenism, ovary dysfunction, polycystic ovarian morphological abnormalities, chronic ovulation, amenorrhea, hirsutism, thyroid dysfunction, hyperprolactinemia, androgen-secreting neoplasms, and nonclassic adrenal hyperplasia. There is substantial evidence that in polycystic ovarian syndrome, metformin decreases weight gain, improves insulin resistance, and restores menstruation, suggesting a possible link between the metabolic abnormalities and hyperprolactinemia and amenorrhea in polycystic ovarian syndrome (
17–
19).
Because polycystic ovarian syndrome is a common disorder, it would be expected to be equally common in schizophrenia, and it is possible that polycystic ovarian syndrome or pre-polycystic ovarian syndrome exists in many of these patients and is involved in antipsychotic-induced weight gain. There are clearly analogies between polycystic ovarian syndrome and antipsychotic-induced weight gain and amenorrhea. Metformin increases dopaminergic tone in polycystic ovarian syndrome (
23), which would reduce the patient's elevated prolactin level. Even so, the exact mechanism by which metformin restores menstruation in polycystic ovarian syndrome is not clearly understood, again because of metformin's many actions. Since most patients with polycystic ovarian syndrome are obese and have consequent metabolic problems, metformin clearly restores menstruation and decreases weight in these patients, but it can also restore menstruation in normal-weight patients with polycystic ovarian syndrome (
35). We found here that the restoration of menstruation was strongly related to weight loss and that weight loss preceded the normalization of other metabolic disturbances such as insulin resistance, which is similar to the circumstances of restoration of menstruation in women with polycystic ovarian syndrome (
19). The menstrual determinants in antipsychotic-induced elevated prolactin levels, weight gain, and metabolic changes are disassociated in that the drugs that cause the most weight gain (olanzapine and clozapine) do not elevate prolactin levels, while sulpiride, amisulpride, and high-potency first-generation antipsychotics do, but only risperidone causes moderate weight gain and elevated prolactin levels.
Since the relationship between menstruation restoration and weight loss is stronger than the relationships between menstruation restoration and prolactin decrease or insulin resistance index normalization, it will require further research to disentangle the relative roles of these various changes in menstruation restoration and antipsychotic-induced amenorrhea.
Weight gain, metabolic abnormalities, prolactin elevation, and amenorrhea are important side effects of antipsychotics. Metformin provides a new option for managing these complications. Indeed, amenorrhea, weight gain, and metabolic syndrome can occur in the same patient, so metformin may be a particularly important potential treatment, especially for those who have a good therapeutic response to risperidone. Of course, a patient with amenorrhea on risperidone could be switched to olanzapine or clozapine and have a good clinical response, but those drugs may induce further weight gain.
We found previously (
16) that substantial weight loss can occur with metformin. Indeed, the average weight loss with metformin coupled with lifestyle changes was 4.7 kg, whereas weight increased by 3.1 kg in placebo-treated patients. The insulin resistance index improved markedly (a decrease of 3.6) in the metformin-plus-lifestyle-changes group, but deteriorated (an increase of 0.4) in the placebo group, with similar changes in fasting glucose and insulin levels, although Western studies do not all show a large change. Metformin has been used widely for the past 50 years throughout the world for type 2 diabetes and is considered by many guidelines to be the drug of choice for this indication because it reduces weight and has a good safety profile (
13). Some consider it the treatment of choice for polycystic ovarian syndrome-induced amenorrhea (
19). Rarely lactic acidosis can occur, but usually in the context of either metformin overdose or medical contraindications (
19). In a study of the accumulated experience of more than 30,000 patients, no cases of lactic acidosis were reported (
36). In the present study, we observed no adverse effects of metformin on the positive and negative symptoms of schizophrenia. Long-term therapy with metformin is associated with decreased intestinal absorption of vitamin B
12 and folate, although in adults the reductions in these nutrients are rarely clinically important, and they are reversed when the drug is discontinued (
37).
Our study has some limitations. First, this was a 6-month trial, and therefore we do not know whether restoration of menstruation and reduction of body weight can be sustained after patients stop taking metformin. Second, we did not monitor serum B12 and folate levels, although our laboratory tests did indicate that there was no decline in hemoglobin levels in any of the patients. Third, we did not examine pharmacokinetic interactions between antipsychotics and metformin. However, metformin is unlikely to have interactions with antipsychotics because it is not metabolized and does not inhibit the metabolism of other drugs. Fourth, our sample size was relatively small. Finally, our patients, as first-episode schizophrenia patients with less than a year of illness, were young, so we do not know whether metformin would have the same effect in older or chronic patients with schizophrenia.
Despite these limitations, this study has shown clearly that the addition of metformin to antipsychotics is a potential treatment to restore antipsychotic-induced amenorrhea in female patients with schizophrenia.