Converging lines of evidence suggest that the cholinergic system may be a target for the development of novel molecular approaches for the treatment of depression (
1). First, cholinergic hyperactivity plays a role in the pathophysiology of depression (
2–
4) and can exacerbate depression-like symptoms in patients and comparison subjects (
4–
6). Second, tobacco dependence is highly prevalent in patients with major depressive disorder (
7), and depressed patients have a high prevalence of smokers (
8–
10). Population-based studies show that smokers are twice as likely as nonsmokers to have a lifetime history of major depression (
11–
13). Furthermore, nicotine binds to neuronal nicotinic acetylcholine receptors (nAChRs), and partial agonists and antagonists of nAChRs, including nicotine (
14), mecamylamine and dihydro-β-erythroidine (
15), cytisine (
16), and varenicline (
17), have antidepressant-like effects in preclinical studies. However, mice lacking the β
2 nAChR subunit are insensitive to the antidepressant-like effects of mecamylamine (
15) and amitriptyline (
18). This suggests that β
2-subunit-containing (β
2*) nAChRs may be important for the antidepressant-like effect in preclinical models of depression. In addition to nicotine (
19,
20), nAChR modulators have been used successfully in clinical trials. Mecamylamine (
21), the S-enantiomer of mecamylamine (
http://clinicaltrials.gov/ct2/show/NCT00593879), and varenicline (
22) have been shown to have antidepressant effects in depression.
The development and use of [
123I]5-I-A-85380 ([
123I]-5-iodo-3-[2(S)-2-azetidinylmethoxy] pyridine) as a radioligand in single photon emission computed tomography (SPECT) to quantify β
2*-nAChRs in vivo in the human brain now allow unparalleled access to this system in vivo (
23–
25). The objective of our study was to determine whether there is a core dysfunction in the β
2*-nAChR system in depression, as assessed by using SPECT and the selective β
2*-nAChR radioligand [
123I]5-I-A-85380. We chose to study subjects who were fully recovered from major depression in order to avoid the confounding effects of acute illness or antidepressant treatment (
26). We hypothesized that patients with major depressive disorder would have lower β
2*-nAChR availability than healthy comparison subjects.
As in radioligand-based studies of dopamine in humans, it is critically important to note that changes in receptor availability in vivo could be due to a change in receptor number or a change in receptor occupancy. It has been shown previously in nonhuman primates that elevated acetylcholine levels induced by a high dose of intravenous physostigmine, an acetylcholinesterase inhibitor, can compete effectively in vivo with [
123I]5-I-A-85380 binding at β
2*-nAChRs (
27). Therefore, lower binding of [
123I]5-I-A-85380 to the β
2*-nAChR could reflect one of two processes: an actual, absolute change in the number of receptors or a change in synaptic acetylcholine levels, such that an increased amount of acetylcholine would prevent the radioligand from binding to the receptor, resulting in low binding of the radioligand.
We also quantified β2*-nAChR availability in postmortem samples of brains from depressed patients and healthy comparison subjects. In the absence of endogenous acetylcholine, β2*-nAChR availability in the postmortem human samples would help interpret the precise mechanism of potential binding abnormalities observed with [123I]5-I-A-85380 in humans.
Method
SPECT Study
Participants.
Each prospective subject had an interview with an experienced psychiatrist, who elicited a complete psychiatric and medical history and conducted a Structured Clinical Interview for DSM-IV Disorders (SCID-I) (
28), standardized psychiatric assessments, and a physical examination. Routine blood tests, a pregnancy test, urine toxicology, and ECG were also performed. In addition, the participants were given the Beck Depression Inventory (BDI) (
29), the Center for Epidemiologic Studies Depression Scale (CES-D Scale) (
30), the Spielberger State-Trait Anxiety Inventory (
31), the NEO Personality Inventory–Revised (
32), the Childhood Trauma Questionnaire (
33), and the 21-item Hamilton Depression Rating Scale item (HAM-D) (
34).
The study participants were 23 nonsmoking, medication-free subjects with recurrent major depressive disorder and 23 age- and gender-matched healthy comparison subjects. Eight of the subjects with major depression were acutely ill, and 15 were fully recovered. Subjects with any other significant axis I diagnosis were excluded from the study, and only those with a primary diagnosis of major depressive disorder were included. In accordance with previous studies of depressed subjects (
35–
37), all of those in the current study had 1) an onset of the first major depressive episode before age 25 years, 2) a lifetime history of at least two major depressive episodes, and 3) at least one first-degree relative with a reported history of major depressive disorder or an axis I disorder.
The depressed subjects had been medication-free for at least 3 months, and the acutely ill subjects scored above 16 on the HAM-D. Subjects were classified as recovered from major depression on the basis of four criteria: self-reported euthymia for more than 4 months following the last episode of major depression, clinician-rated euthymia for more than 4 months according to a clinical interview, absence of criteria for a major depressive episode for more than 4 months as judged with the SCID-I, and a HAM-D score of less than 8. Comparison nonsmoking subjects were included in the study only if they had no lifetime history or family history (in a first-degree relative) of any axis I or axis II disorder as judged with the SCID-I.
None of the subjects recruited had smoked for at least 6 months, and 14 of the depressed subjects (six acutely ill and eight recovered) reported no lifetime history of smoking. Nonsmoking status was confirmed by a plasma cotinine level less than 15 ng/ml, a urine cotinine level less than 100 ng/ml (confirmed by using a dipstick urine test on the day of the scan), and exhaled carbon monoxide level less than 11 ppm on the day of intake and on the day of the scan. Subjects were excluded if they had a positive pregnancy test on the day of the SPECT scan or prior radiation exposure within the past year such that participation in this study would place them over the U.S. Food and Drug Administration (FDA) limit for annual radiation exposure.
This study was approved by the Yale University School of Medicine Human Investigation Committee and the Radiation Safety Committee. After complete description of the study to the subjects, written informed consent was obtained. The use of the radiotracer [123I]5-I-A-85380 was approved by the FDA.
Imaging.
Each subject had one [
123I]5-I-A-85380 SPECT scan and one magnetic resonance imaging (MRI) scan. MRI was performed on a Signa 1.5-T system (General Electric, Milwaukee, Wis.) as described previously (
38). SPECT imaging was conducted as described previously (
39). In brief, [
123I]5-I-A-85380 was administered by using a bolus plus constant infusion at a ratio of 7.0 hours (i.e., with a bolus worth 7 hours of infusion) for 8 hours. There were no significant differences in the total injected dose, bolus dose, infusion dose, or bolus-to-infusion ratio between the depressed and comparison groups (
Table 1). Three 30-minute emission scans and one 15-minute simultaneous transmission and emission protocol (STEP) scan were obtained between 6 and 8 hours of the infusion on a Picker PRISM 3000 XP SPECT camera (Picker, Cleveland). Plasma samples were collected in the middle of the second scan to quantify the total amount of parent tracer and the free fraction (f
p) in plasma (
40) and to correct for individual differences in metabolism and protein binding of [
123I]5-I-A-85380 (
41). A
57Co-distributed source was measured with each experiment to control for day-to-day variation in camera sensitivity.
Image analysis and outcome measures.
SPECT emission images were analyzed as described previously (
25). Specifically, SPECT emission images were reconstructed by using a filtered-back projection algorithm with a ramp filter on a 128×128 matrix to obtain 50 slices with a pixel size of 2.06×2.06×3.56 mm in the x, y, and z axes. A three-dimensional Butterworth filter (order 10, cutoff frequency 0.24 cycle/pixel) was applied post hoc. A coregistered magnetic resonance (MR) image was used to guide the placement of standard two-dimensional region-of-interest templates by means of MEDx software (Medical Numerics, Germantown, Md.). A three-dimensional volume of interest was generated for each region and transferred to the coregistered SPECT image to determine regional radioactive densities. The chosen regions were those known to contain β
2*-nAChRs and included the frontal, parietal, anterior cingulate, temporal, and occipital regions, the thalamus, the striatum (an average of caudate and putamen), the hippocampus, the amygdala, the brainstem, and the cerebellum. Regional [
123I]5-I-A-85380 uptake was determined by V
T/f
p, where V
T is the total volume of distribution and f
p is the free plasma fraction. Each case was analyzed by two raters, and the mean of the two raters' values was used. Interrater variability for V
T/f
p was less than 10% across all regions and was computed as the percentage difference between the two raters by means of the following equation: [(V
T/f
p1 / V
T/f
p2)–1]×100=% difference.
Voxel-Based Morphometric Analysis
In order to study any volumetric differences, as previously reported in patients with major depression (
42,
43), a voxel-based morphometric analysis of the data was performed with SPM5 (Wellcome Trust Centre for Neuroimaging, London). A detailed description of the steps involved in processing data for voxel-based morphometry is available in the SPM5 manual (
http://www.fil.ion.ucl.ac.uk/spm/). Before preprocessing, the images were checked for movement artifacts and the origin of each image was set at the anterior commissure. The images were segmented, normalized, and smoothed simultaneously by means of a unified segmentation algorithm (
44). In contrast to optimized voxel-based morphometry, which was used in SPM2 and in which these steps were completed sequentially, this study used the unified segmentation algorithm in SPM5 to simultaneously calculate image registration, tissue classification, and bias correction by using our participants' structural MR images combined with the tissue probability maps provided in this version of SPM. Structural MR images were segmented into gray matter, white matter, and CSF. The segmented and modulated normalized images were smoothed with an 8-mm full-width-half-maximum filter. A two-sample t test was then used to delineate any significant differences in gray matter volume between the depressed and healthy comparison groups (N=23 each), between the recovered depressed and matched comparison groups (N=15 each), and between the acutely depressed and matched comparison groups (N=8 each) separately. Given the small number of subjects and the preliminary nature of this analysis, a threshold of p<0.05, uncorrected, was used to view the results.
Postmortem Study
Human brain specimens from the prefrontal cortex of 14 depressed subjects and 14 age-matched comparison subjects were obtained from the Dallas Brain Collection (
45). Methods are described in the data supplement accompanying the online version of this article.
Statistical Analysis
All data were analyzed by using SAS version 9.1 (SAS Institute, Cary, N.C.). Before analysis, the data were examined descriptively and tested for normality by using normal probability plots and Kolmogorov test statistics. All data were approximately normal. Receptor binding data were analyzed by means of linear mixed models. These models included region as a within-subjects factor, diagnostic group (acutely depressed, recovered depressed, and respective comparison subjects) as a between-subjects factor, and the interaction between group and region. On the basis of the Bayesian information criterion, the best-fitting variance-covariance structure was compound symmetry with heterogeneous variance—i.e., a constant correlation between regions and region-specific variances are estimated. Significant interactions were explained by appropriate post hoc tests and graphical displays. Post hoc tests were adjusted for the 11 regional comparisons by using the Bonferroni correction (i.e., a two-sided alpha of 0.0045 was used as the threshold). An omnibus model including the four diagnostic groups was first developed. After a significant group-by-region interaction was confirmed, separate models were developed for 1) all depressed patients versus all comparison subjects, 2) acutely ill patients versus respective comparison subjects, 3) recovered patients versus respective comparison subjects, and 4) acutely ill versus recovered depressed patients. Inclusion of age and gender as covariates did not alter the results, and they were therefore dropped from the analysis. Potential associations between binding potential and crucial clinical variables of interest were assessed by using correlation analysis. In order to control for multiple correlations, the correlations of clinical and personality variables with binding data were reported only where significant correlations were observed consistently in a region. Postmortem data were analyzed by using analysis of variance (ANOVA), and correlations were analyzed by using appropriate correlation coefficients. For these data, each cell was an average of three measurements performed for each brain region for each patient. The data were then analyzed by ANOVA with group (comparison or depressed) as a between-subjects factor and brain region (gray or white matter) as a within-subjects factor. Alpha was set at 5%.
Results
SPECT Study
Subject characteristics.
Clinical and demographic characteristics of the participants in each group are shown in
Table 2; the groups were well matched on most variables studied, although there were significant differences in ethnicity and lifetime smoking history among the groups. Consistent with the acute nature of their illness, the subjects in the acute depression group had significantly higher scores on the CES-D scale, the BDI, the NEO Personality Inventory neuroticism subscale, the Childhood Trauma Questionnaire total scale and physical neglect subscale, and the Spielberger State-Trait Anxiety Inventory and significantly lower scores on the NEO Personality Inventory conscientiousness subscale than the subjects recovered from major depression.
β2*-nAChR availability in major depressive disorder.
The radiochemical blood measures are shown in
Table 1.
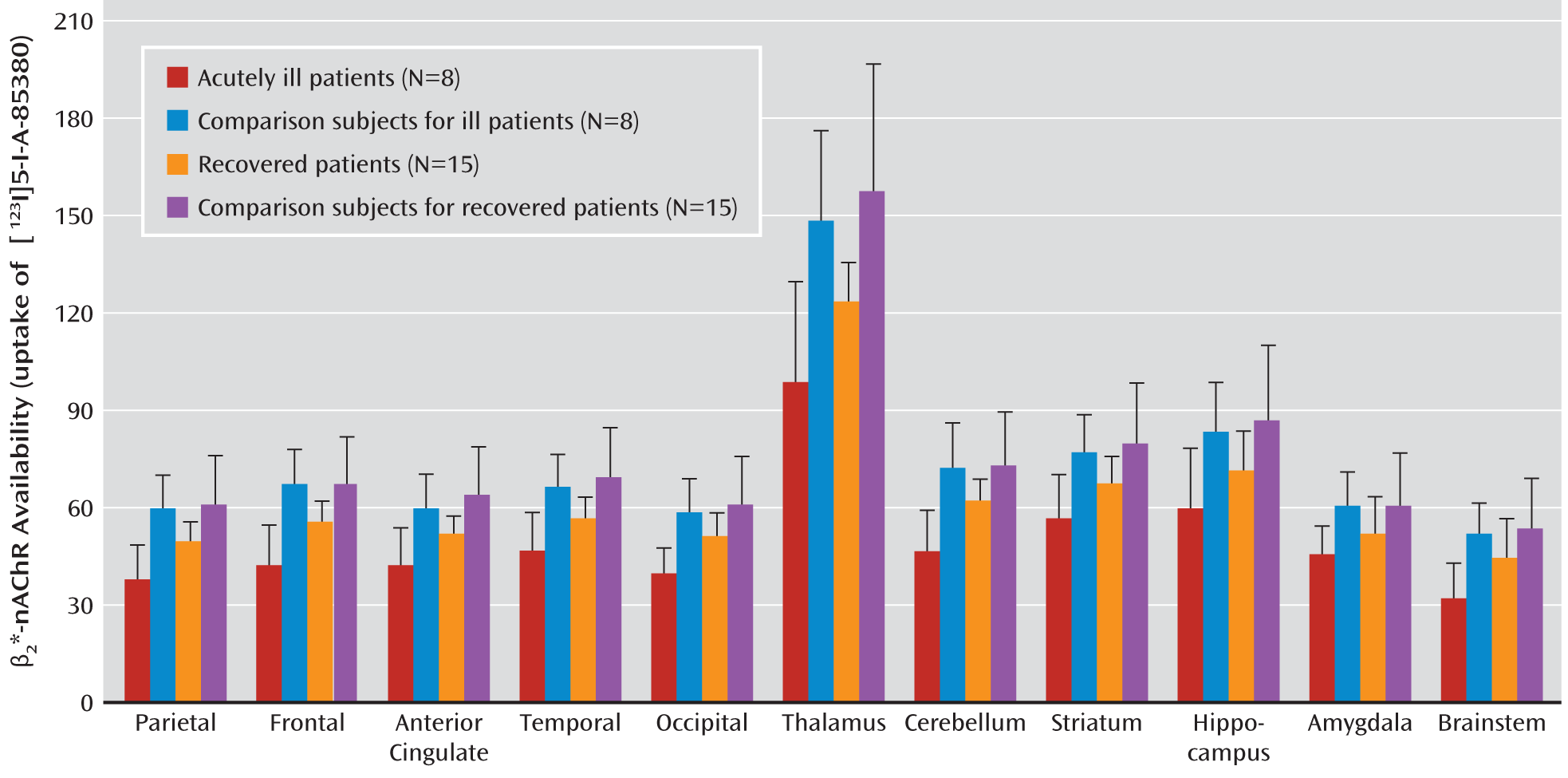
Figure 1 presents β
2*-nAChR availability by region. The results of the omnibus model including all four diagnostic groups revealed a significant effect of group (F=8.7, df=3, 42, p<0.0001) and a significant group-by-region interaction (F=1.8, df=30, 420, p=0.006). Given the significant interaction, we chose to disentangle the group-by-region differences by developing independent models including two groups at a time.
In the comparison of acutely depressed patients and their comparison subjects, there was a significant main effect of group (F=15.8, df=1, 14, p=0.002) and a significant interaction between group and region (F=2.5, df=10, 140, p=0.008). Post hoc comparisons revealed lower β2*-nAChR availability in the acutely ill depressed subjects than in the healthy comparison subjects in nine of the 11 regions of interest examined (for all regions except the hippocampus and amygdala, adjusted p<0.007).
In the comparison of the recovered depressed patients and their matched comparison subjects, there was also a significant group effect (F=7.9, df=1, 28, p=0.009) and group-by-region interaction (F=2.0, df=10, 280, p=0.03). Post hoc comparisons showed lower β2*-nAChR availability among patients than among comparison subjects in the frontal cortex (adjusted p=0.03), anterior cingulate (adjusted p=0.04), and thalamus (adjusted p=0.008).
When we compared the acutely ill and recovered depression patients, we found a significant overall group effect (F=10.0, df=1, 21, p=0.005), with lower overall β2*-nAChR availability in the acutely depressed patients than in those who were recovered. However, the interaction between group and region was not significant (F=1.3, df=10, 210, p=0.21).
Correlation of β2*-nAChR availability with clinical features.
The analysis of lifetime number of episodes included 19 patients (four acutely ill, 15 recovered). There was a significant positive correlation between lifetime number of episodes and β2*-nAChR availability in the temporal cortex (r=0.46, p<0.05), occipital cortex (r=0.48, p=0.04), and striatum (r=0.49, p=0.04). This was also observed in the recovered group alone (N=15) in the temporal cortex (r=0.54, p=0.04), occipital cortex (r=0.56, p=0.03), thalamus (r=0.54, p=0.04), and striatum (r=0.58, p=0.03).
In the patients with acute depression but not the recovered group, the scores on the total Childhood Trauma Questionnaire and its emotional abuse subscale were negatively correlated with receptor availability in all regions of interest studied except the amygdala. The score on the trait subscale of the Spielberger State-Trait Anxiety Inventory was negatively correlated with receptor availability in the anterior cingulate (r=–0.65, p=0.03), temporal (r=–0.64, p=0.03), and occipital (r=–0.65, p=0.03) regions and showed a nearly significant correlation in the striatum (r=–0.51, p=0.09) in the recovered group but not in the acutely ill patients. The score on the NEO Personality Inventory conscientiousness subscale was significantly correlated with receptor availability in all regions of interest studied except the amygdala and brainstem (which showed a nearly significant correlation) in the acutely ill group but not the recovered group.
The relationship between a history of past cigarette smoking and β2*-nAChR availability could not be studied because of the small and unbalanced number of subjects with a past history of cigarette smoking.
Voxel-Based Morphometric Analysis
An SPM analysis of brain volumes of all depressed patients in relation to all comparison subjects showed no significant differences in volume, even at the relatively generous threshold used in the analysis. Similarly, there were no significant differences in volume between the acutely ill depressed subjects and their respective comparison subjects, between the recovered depressed subjects and their respective comparison subjects, or between the acutely ill and recovered depressed subjects.
Postmortem Study
Demographic data for the patients with a diagnosis of major depressive disorder and the matched comparison subjects and the results of [125I]5-I-A-85380 equilibrium binding in postmortem brain tissue are provided in the data supplement accompanying the online version of this article. There were no statistically significant differences in β2*-nAChR number between the depressed patients and the healthy comparison subjects. Both the main effect of group (F=0.46, df=1, 23, p=0.52) and the interaction of region and group (F=0.23, df=1, 46, p=0.78) were nonsignificant.
Discussion
This multimodal analysis of β
2*-nAChR availability shows that major depressive disorder is associated with significant dysfunction in the cholinergic system. From the in vivo data, we observed that acutely ill and recovered patients with major depression had significantly lower β
2*-nAChR availability than age- and gender-matched comparison subjects. Crucially, there were no differences in β
2*-nAChR number between the postmortem samples of depressed and comparison subjects under conditions that washed out any endogenous bound acetylcholine. This suggests that the lower β
2*-nAChR availability in vivo is likely better explained by high levels of extracellular acetylcholine in patients with major depression than by a low total number of receptors. This would be consistent with the cholinergic hypothesis of depression (
4). Finally, we found that the lower β
2*-nAChR availability in depressed patients had functional effects in that it was related to critical personality measures.
Use of [123I]5-I-A-85380 to Quantify β2*-nAChR Availability
Our data demonstrate a persistent enduring deficit in cortical and subcortical β
2*-nAChR availability in subjects with major depressive disorder. As the acutely ill patients had significantly lower β
2*-nAChR availability than the recovered subjects in most regions, it is possible that acetylcholine levels recover somewhat in patients with remitted depression. The continued significantly lower β
2*-nAChR availability in fully recovered, euthymic medication-free subjects with depression suggests that the changes in depression are not an epiphenomenon of treatment or illness but may be associated with trait vulnerability to depression, as demonstrated by us previously for other targets (
26,
46,
47). nAChRs have a ubiquitous role in the modulation of multiple neurotransmitter systems considered crucial in the pathophysiology of depression, such as serotonin, noradrenaline, glutamate, and GABA (
48), and the observed dysfunction may reflect a cause or effect of dysfunction in these other systems. Finally, there were no differences in brain volume between the patients and comparison subjects, suggesting that brain atrophy could not account for differences in receptor availability in this study group.
Postmortem Studies
As described in the preceding, the lower binding of the SPECT radioligand [
123I]5-I-A-85380 to β
2*-nAChRs could reflect an actual, absolute abnormality in the number of receptors or it could reflect a difference in synaptic acetylcholine levels, with a greater amount of acetylcholine preventing the radioligand from binding to the receptor (
27). Consistent with the latter possibility, the postmortem samples showed no difference in receptor number between the depressed patients and either comparison group. These results, together with the nonhuman primate study (
27), suggest that when β
2*-nAChR availability is measured in the absence of bound endogenous acetylcholine, there is no evidence of an abnormality in receptor number.
Functional Consequences of Lower β2*-nAChR Availability
We observed a significant positive correlation between the number of depressive episodes and β
2*-nAChR availability in the combined depressed group and in the recovered patients. If we assume that the lower β
2*-nAChR availability represents higher extracellular acetylcholine levels, the correlation implies that the greater the number of episodes, the greater the amount of extracellular acetylcholine. Studies suggest that tobacco smokers have higher β
2*-nAChR availability than do nonsmokers in nonpsychiatric populations (
39) and that it normalizes after 6–12 weeks of abstinence (
49). However, given the small and unbalanced number of subjects with a past history of cigarette smoking, we were not able to determine the effect of this variable on β
2*-nAChR availability. Finally, the association between childhood trauma and β
2*-nAChR availability replicates the relationship between trauma and major depressive disorder that has been observed in previous studies (
50).
Limitations
Notwithstanding the robust statistical significance of the SPECT binding results, the study group was relatively small and composed nearly entirely of subjects without a past history of cigarette smoking; only 11 of the 46 subjects studied had a past history of cigarette smoking. Future studies need to be performed to examine the relationship between past cigarette smoking and β
2*-nAChR availability. The nature and logistics of using a SPECT radioligand, the absence of a quantifiable reference region and input function, and the absence of a measurement of nondisplaceable binding in patients with major depressive disorder are issues that are beyond the scope of this article but have been addressed in detail elsewhere (
25,
27,
51). While the amygdala is a crucial structure in the pathophysiology of depression, SPECT has poor resolution for small brain regions and the findings for the amygdala should be interpreted with caution. A PET study needs to be performed to confirm these results. Although past smoking status is a critical issue, it was not possible to analyze this effect adequately given the small and unequal number of subjects who have smoked in the past. The use of a population exclusively composed of lifetime nonsmokers limits generalizability; however, it is essential to interpretation of the results and should be examined in future studies.
Conclusions
Patients with major depressive disorder show lower β
2*-nAChR availability that persists beyond the cessation of treatment and into full recovery. Further studies are needed to clarify the molecular underpinnings of this low receptor availability. A similar study using a SPECT ligand specific to β
4-containing nAChRs seems warranted because of the high density of these receptors in the habenula complex, a region increasingly implicated in depressed mood states (
52).