Obsessive-compulsive disorder (OCD) is a debilitating psychiatric disorder affecting 2%–3% of the general population (
1) that is characterized by time-consuming obsessions (repetitive intrusive thoughts) and compulsions (repetitive ritualistic behaviors). OCD has a partially genetic etiology, with twin studies indicating a heritability of OCD symptoms in adults ranging from 27% to 45% and first-degree relatives of patients having four times the risk for developing OCD symptoms relative to family members of comparison subjects (
2). Genome-wide linkage studies of OCD suggest susceptibility loci on several chromosomes (
3), which is in line with the supposed involvement of multiple genetic polymorphisms related to dopaminergic, serotonergic, and glutamatergic neurotransmission and neuronal outgrowth and myelination (
2,
4). Like most neuropsychiatric disorders (
5), OCD has a complex genetic background, with probably several environmental and epigenetic factors interacting with multiple genes to give rise to a heterogeneous phenotype. Phenotypical categorization into OCD subdimensions has only been partially useful to the genetic study of OCD (
6,
7).
To obtain a better understanding of the etiological underpinnings of OCD, therefore, a search for endophenotypes of the disorder is warranted (
8). An endophenotype is “a measurable trait along the path between phenotype and distal genotype, reflecting a simpler clue to the genetic basis of a disorder than the syndrome itself, and thus can help clarify the exact genetic contributions to a disease” (
5). One candidate endophenotype of OCD is impaired response inhibition as measured by the stop-signal task (
8). Response inhibition is the process by which a motor action is withheld upon the appearance of a stop signal. Problems with inhibition are apparent in the phenomenology of OCD, with patients seemingly unable to stop their obsessions and compulsions. Indeed, patients with OCD and their first-degree relatives share a lengthened duration of the inhibitory process—i.e., their stop-signal reaction time is elevated (
9,
10). A meta-analysis (
11) has shown that this inhibition deficit may be a more prominent feature of OCD when compared with other related disorders such as Tourette’s syndrome or anxiety and mood disorders.
Response inhibition is associated with a core network comprising the presupplementary motor area, the inferior frontal gyrus, and the subthalamic nucleus (
12–
14). There is, however, also robust evidence for the involvement of the inferior parietal cortex in inhibition from studies in healthy subjects and patient groups showing inhibition impairments (
10,
14–
18). The exact role of the above-mentioned regions in the subsequent steps of response inhibition (i.e., attentional monitoring of the stop signal, resolving the response conflict, and inhibiting a prepotent motor response) is still up for debate (
12,
14). The behavioral inhibition deficit in OCD patients and their first-degree relatives is associated with widespread gray matter density increases in cingulo-parietal regions and decreases in frontal regions (
10). Furthermore, a diffusion tensor imaging study of the same sample of patients and relatives (
19) indicated white matter integrity changes with a similar spatial pattern in the right inferior parietal cortex and the medial frontal cortex. It remains unclear to what extent these morphological changes are functionally related to the inhibition deficit seen in OCD patients and relatives.
Neurobiological models of OCD have implicated dysfunctional frontal-striatal loops in the etiology of clinical symptoms and cognitive deficits (
20). An imbalance between hypoactive brain areas associated with cognitive control and hyperactive brain areas associated with the processing of errors (i.e., the anterior cingulate cortex) is thought to underlie the inhibitory dysfunction and increased error sensitivity found in OCD patients (
20–
22). This frontal-striatal model of OCD was revised as the involvement of other regions such as the parietal cortex became increasingly clear (
20). The four functional neuroimaging studies conducted to date on response inhibition in OCD have produced inconsistent results. Task-related activity in the inferior frontal gyrus (
23–
26) and the premotor cortex (
24,
26), possibly extending to the presupplementary motor area, and anterior cingulate cortex (
23,
26) was found to be both increased and decreased in patients relative to comparison subjects. These inconsistent findings may result from methodological constraints such as limited power, age differences, and medication confounds.
Method
Participants
Forty-five medication-free patients with OCD, 17 of their siblings, and 39 healthy comparison subjects participated in the study (see
Table 1 for a summary of demographic characteristics). Four patients and two comparison subjects were excluded from the analysis for various reasons (see section S1.1 in the
data supplement that accompanies the online edition of this article). Patients and siblings were recruited through outpatient clinics within the Netherlands OCD Association consortium (
http://nocda.amstad.nl) and the Academic Anxiety Center Altrecht (Utrecht, the Netherlands) and by online advertisements. Comparison subjects were recruited by local and online community advertisements.
We screened all participants for axis I psychiatric disorders with the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (
27). OCD symptom characteristics and severity were assessed with the Obsessive-Compulsive Inventory–Revised (
28) and the Yale-Brown Obsessive Compulsive Scale (
29), respectively. Participant mood was assessed with the Montgomery-Åsberg Depression Rating Scale (MADRS) (
30), and handedness was assessed with the Edinburgh Handedness Inventory (
31). All participants were between 18 and 65 years old and had normal or corrected-to-normal vision.
Exclusion criteria were psychoactive medication use, current or past psychosis, a major physical illness, a history of a major neurological illness, or MRI contraindications. The OCD patients were medication free for at least 4 weeks, and they could participate if they had a primary diagnosis of OCD without predominant hoarding. Psychiatric comorbidity (including tic disorders) was not an exclusion criterion. Siblings did not meet criteria for OCD, and comparison subjects had no current DSM-IV-TR axis I diagnosis and no family history of OCD. All procedures were approved by the local ethical review board, and all subjects provided written informed consent.
Stop-Signal Task
The participants performed a visual stop-signal task (
32) during functional MRI (fMRI), in which they indicated the direction of an arrow with a button press. Eighty percent of the trials were simple go trials. Unpredictably, on 20% of the trials, a stop signal (cross) appeared with a delay after the arrow. This stop-signal delay was updated such that the stop-success-to-error ratio was approximately 50%. Behavioral outcome measures were the subject-specific stop-signal reaction time (speed of the stop process), the mean reaction time on accurate go trials (speed of go process), and the error percentage on go trials (overall attention; see section S1.2 of the online
data supplement for further task specifications).
Behavioral Analyses
We analyzed the group effects on demographic, clinical, and behavioral measures by one-way analysis of variance (ANOVA) with SPSS, version 15 (Chicago, 2006). Statistical significance was set at p<0.05, two-tailed. Significant group interactions and trends (0.05≤p≤0.10) were followed up by post hoc two-sample t tests. If data did not meet parametric assumptions, we used nonparametric tests as indicated. We assessed group effects on gender and handedness with chi-square tests. To assess whether clinical variables (i.e., disease severity and MADRS scores for patients and Obsessive-Compulsive Inventory–Revised scores for siblings) influenced task performance, we performed correlation analyses.
Image Acquisition
Imaging was done on a GE Signa HDxt 3-T MRI scanner (General Electric, Milwaukee) at the VU University Medical Center. Functional images were acquired with a gradient echo-planar imaging sequence (TR=2,100 ms; TE=30 ms; 64×64 matrix; field of view=24 cm; flip angle=80°) with 40 ascending slices per volume (3.75×3.75 mm in-plane resolution; slice thickness=2.8 mm; interslice gap=0.2 mm), which gave whole-brain coverage. Structural scanning included a sagittal three-dimensional gradient-echo T1-weighted sequence (256×256 matrix; voxel size=1×0.977×0.977 mm; 172 sections).
Image Processing and Analyses
Functional images were analyzed with SPM8 (Wellcome Trust Centre for Neuroimaging, London). To minimize motion-related artifacts, images were reoriented and realigned. The high-resolution structural T1 scan was coregistered with the mean functional image and spatially warped to the Montreal Neurological Institute (MNI) T1 template. This normalization matrix was applied to the functional images. Data were resliced with a 3×3×3 mm resolution and spatially smoothed with an 8 mm full width at half maximum Gaussian kernel.
Imaging data were analyzed in the context of the general linear model. At the first level, onsets of accurate go trials, stop-success trials, and stop-error trials were modeled using delta functions convolved with the canonical hemodynamic response function. Participants’ movement parameters were included in the model as regressors of no interest. To remove low-frequency noise, a high-pass filter (128-second cutoff period) was applied.
The inhibition contrast (stop-success trials > go trials) was calculated for each subject to probe blood-oxygen-level-dependent (BOLD) activity related to inhibition. To assess error processing, an error contrast was constructed (stop-error trials > stop-success trials). These two first-level contrast images were brought into second-level random-effects analyses.
A priori hypotheses were tested in specific regions of interest associated with response inhibition and error monitoring, respectively. Using MarsBaR (
http://marsbar.sourceforge.net), 10 mm spherical functional regions of interest were created around the peak voxel coordinates of the main effect of inhibition and error over all subjects (N=95; see Tables S2A and S2B in the online
data supplement) examined at p<0.05, whole-brain family-wise error corrected (
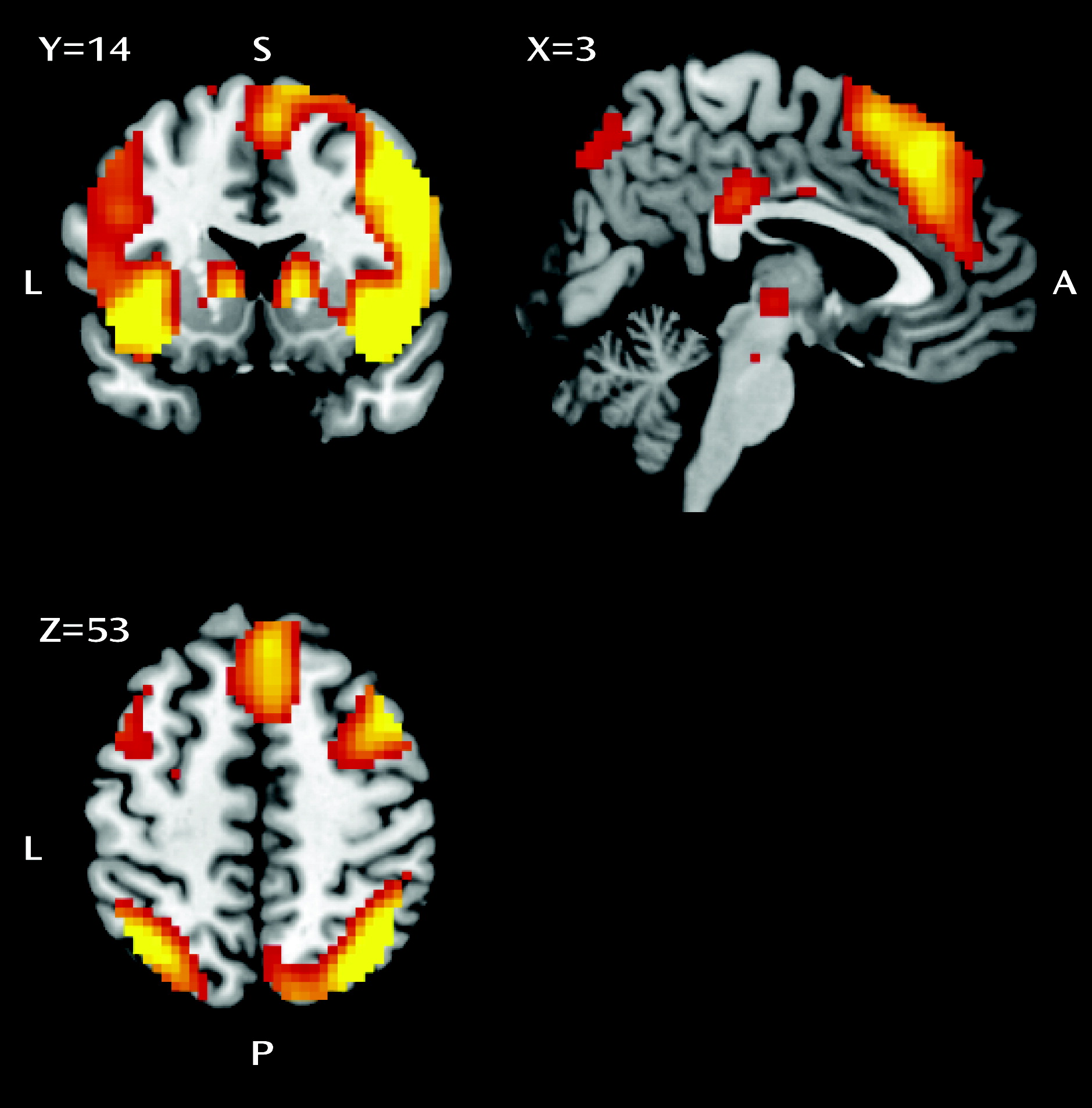
33). The inhibition-related regions of interest were the left and right inferior frontal gyrus (Brodmann’s areas [BAs] 47/45/13; right: x=33, y=23, z=−11; left: x=−33, y=23, z=−8), inferior parietal cortex (BA 40; right: x=42, y=−55, z=43; left: x=−51, y=−55, z=43), presupplementary motor area (BA 6; right: x=9, y=17, z=67; left: x=−15, y=14, z=67), and subthalamic nucleus (x=3, y=−15, z=−2). An error-related anterior cingulate cortex region of interest was constructed around coordinates x=0, y=20, z=34.
Main effects of task were assessed in one-sample t tests per group per contrast. The 41 OCD patients were directly compared with the 37 comparison subjects in a two-sample t test per contrast. To specifically test the 17 patient-sibling pairs, a three-group comparison (one-way ANOVA [N=51]) was performed that also included 17 comparison subjects as a specific age-, gender-, and education-matched comparison group. Group interactions from this ANOVA were followed up by post hoc two-sample t tests. To ensure that group differences in BOLD activity were truly a reflection of genetic susceptibility to OCD, instead of differences in task performance we included stop-signal reaction time as a covariate of no interest in all the analyses. Moreover, because six sibling-patient pairs were discordant for gender, we included gender as a covariate. Imaging results were considered significant when controlled for type I errors for the search volume (p<0.05, family-wise error corrected).
We extracted the peak voxel activity of all clusters of between-group differences. Correlations were then calculated between these values and behavioral (all groups) and clinical measures (within patients and siblings). Results are reported using Pearson’s r unless otherwise indicated.
Discussion
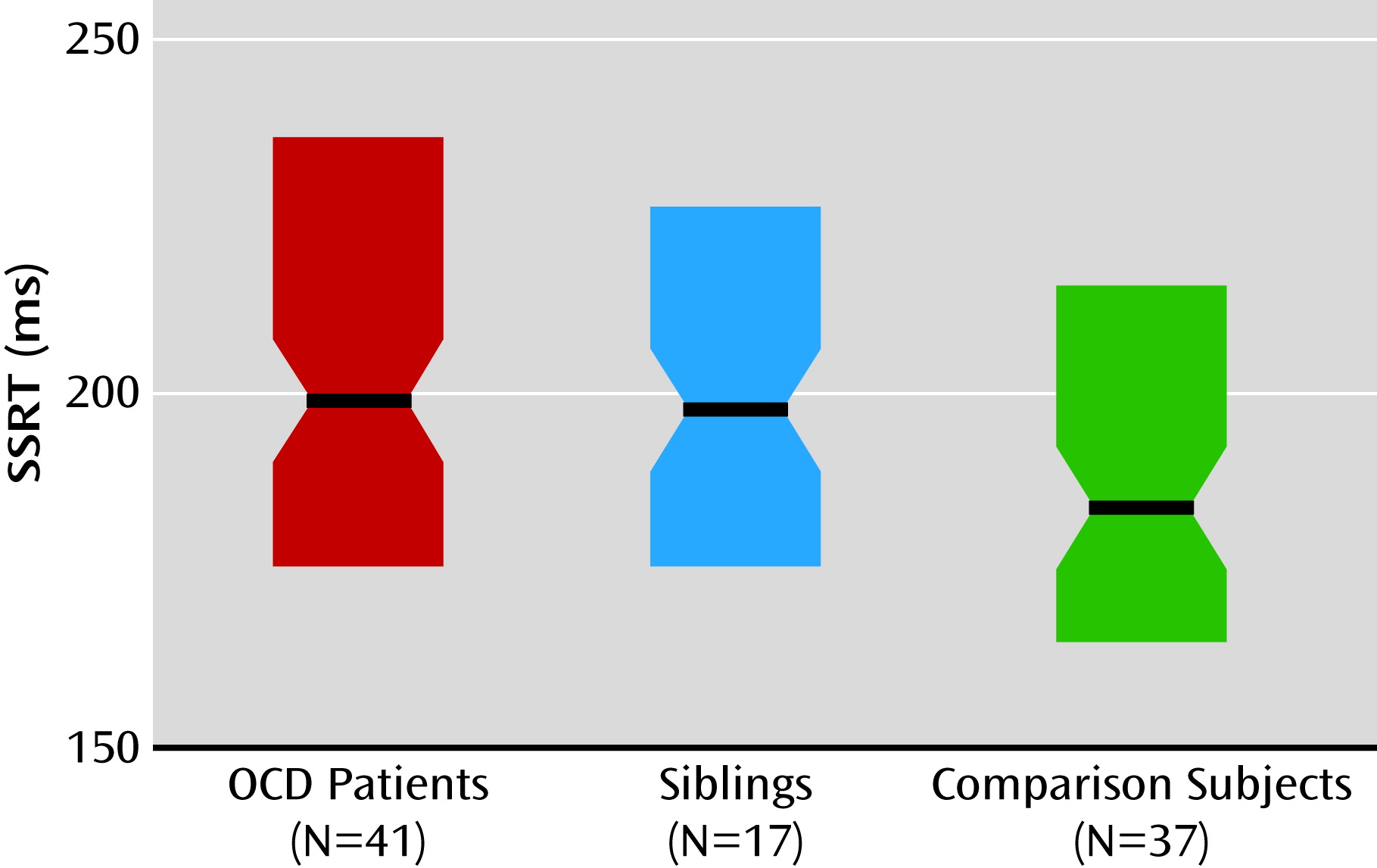
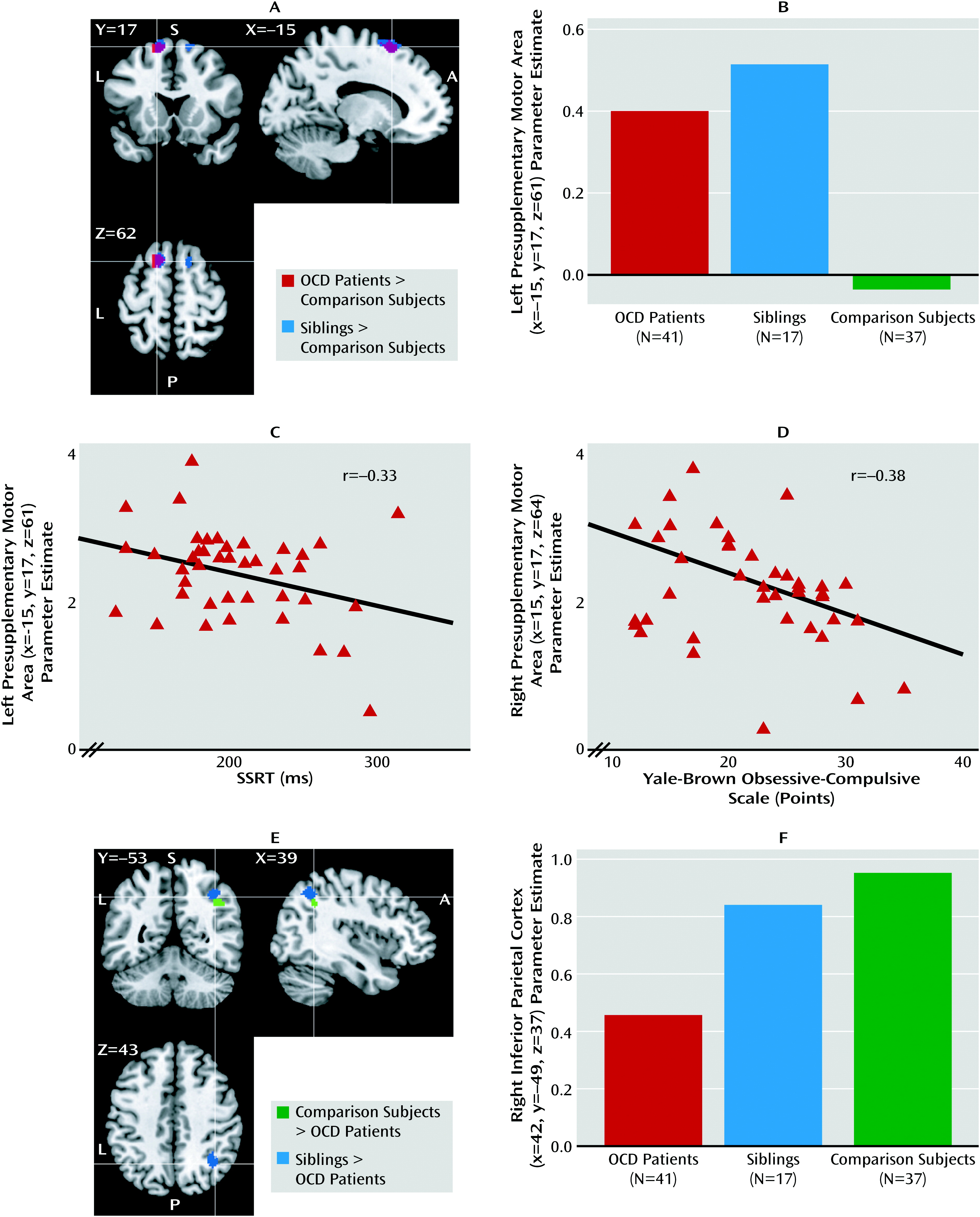
We assessed the functional neural correlates of response inhibition in a large sample of medication-free patients with OCD, their unaffected siblings, and healthy comparison subjects without a family history of OCD. Both patients and their siblings showed behaviorally impaired response inhibition, although for siblings this did not reach statistical significance. Also, both patients and their siblings showed increased recruitment of the presupplementary motor area during inhibition, suggesting that hyperactivity in the presupplementary motor area is a candidate neurocognitive endophenotype of OCD. Moreover, patients showed decreased recruitment of the right inferior parietal cortex and inferior frontal gyrus during inhibition relative to their siblings and the healthy comparison subjects, which possibly contributes to the impairment in response inhibition.
Impaired response inhibition at the behavioral level is consistent with previous studies of OCD patients and their relatives (
9,
10). Left presupplementary motor area hyperactivity in OCD patients and their unaffected siblings during response inhibition is a novel finding. Studies of healthy subjects have provided strong evidence for the key importance of the presupplementary motor area for the rapid resolution of conflicting action plans, which is necessary for adequate stop-signal task performance (
12,
34). The negative correlation between stop-signal reaction time and presupplementary motor area activity in OCD patients and their siblings, with high activity in the presupplementary motor area related to relatively preserved stop-signal task performance, has been reported in healthy subjects (
12,
17,
34). Furthermore, disruption of left presupplementary motor area functioning has been found to result in increased stop-signal reaction time in human participants (
35), and microstimulation studies in monkeys have shown that the presupplementary motor area is critical for inhibition by suppressing the unwanted action and facilitating the desired one (
36). Thus, the presupplementary motor area is important for inhibition in healthy subjects, and we found here that its activity also correlates with performance in OCD patients and their siblings.
Medial frontal cortex abnormalities are an important feature of OCD-related brain changes (
20). Neuroimaging studies (
20,
21,
23,
37,
38) using fMRI, positron emission tomography, and magneto- and electroencephalography have provided evidence for medial frontal hyperactivity in OCD during task performance, which, although most studies focused on changes in the anterior cingulate cortex, sometimes extended to the presupplementary motor area (
39). In addition, biochemical and structural changes in the medial frontal cortex have been observed in patients with OCD and their relatives (
10,
19,
20,
39) and have been found to be correlated with impaired response inhibition (
10). Thus, given the behavioral relevance of presupplementary motor area activity and previous reports of regional structural changes, hyperactivity in patients and siblings may be a compensatory mechanism, with a spreading or increase of regional brain activity reflecting inefficient neural processing of the presupplementary motor area itself. The negative correlation between right presupplementary motor area activity and illness severity in patients further suggests that this compensatory mechanism is failing in severe OCD.
Although we observed no group differences in error monitoring, many studies have associated medial frontal cortex abnormalities in OCD with increased error monitoring (
21,
23,
37,
38). None of these studies, however, showed a clear relationship between brain activation and conflict load or error-related behavior. For instance, results from an EEG study (
38) showing increased error-related negativity after error trials in patients with OCD and their relatives were no longer significant after controlling for baseline amplitude. A recent magnetoencephalography study on error monitoring in patients with OCD instead suggested that the observed anterior frontal cortex hyperactivity was compensatory (
40). Taken together with our results, this suggests that frontal midline hyperactivity in OCD is related to a more general increased compensatory recruitment rather than being specific for error signals. Furthermore, based on the available literature on inhibition and conflict monitoring, we expect that presupplementary motor area hyperactivity during inhibition is specifically present in groups who are at risk for developing OCD and not in non-OCD anxiety (
11,
41). This hypothesis, however, awaits empirical confirmation.
We observed deficient recruitment of the right inferior parietal cortex and inferior frontal gyrus during response inhibition in OCD patients relative to comparison subjects, a finding that was absent in siblings. Hypoactivity in the inferior parietal cortex and inferior frontal gyrus in patients could reflect impaired attention to the stop signal or impaired action reprogramming (
10,
12,
13,
15,
17). We found no evidence for a general attentional deficit in patients, since go trial performance was not affected. Although hypoactivity in the right inferior frontal gyrus was significant only at the subthreshold level, this finding is consistent with previous studies on response inhibition in OCD (
24–
26). Hypoactivity of the right inferior parietal cortex during mental imagery in OCD has been reported (
42); however, this study is the first to observe it during response inhibition.
Abnormalities in parietal cortex functioning in OCD may be related to regional structural brain changes previously observed in patients and relatives that correlated with impaired response inhibition (
10,
19,
20). In our study, however, activity in the inferior parietal cortex (and the inferior frontal gyrus) in siblings was unaffected at the group level, even when explored at a very lenient statistical threshold. We did observe a significant negative correlation between inferior parietal cortex activity and OCD symptom scores in siblings, suggesting that siblings with more subclinical OCD symptoms have a failure of inferior parietal cortex recruitment that is similar to OCD patients. This state dependency of parietal activity in OCD is consistent with the decreased inferior parietal cortex activity during planning observed in twins with high compared with low subclinical OCD symptoms (
43).
The positive correlation in patients between stop-signal reaction time and right inferior parietal cortex activity replicates previous findings of a positive correlation between stop-signal reaction time and inferior parietal cortex activity in healthy subjects and inferior parietal cortex structure in OCD patients and their relatives (
10,
17). Our results further confirm the functional involvement of the inferior parietal cortex in response inhibition. The combination of both inferior parietal cortex hypoactivity in patients and a positive relationship of activity in the inferior parietal cortex with stop-signal reaction time in this group, however, seems counterintuitive, and its explanation is not straightforward. Possibly, increased parietal recruitment during stop-success trials in poor-performing patients reflects an up-regulation of the default mode network, as has been previously suggested (
17). Although the subthalamic nucleus is important for inhibition (
12) and its firing pattern seems dysfunctional in OCD patients (
44), we, like previous OCD inhibition researchers (
23–
26), did not find any group interactions in this region. Future studies should elucidate the exact roles of the inferior parietal cortex and inferior frontal gyrus in inhibitory control and their dysfunction in OCD, and they should replicate our findings.
A limitation of our study is that although gender ratios between the groups were not statistically different, six of the 17 patient-sibling pairs were discordant for gender. Recruiting siblings instead of other first-degree relatives, such as parents, has the benefit of including subjects within a similar age range. Age-dependent effects on response inhibition have been described, so age matching, which was achieved in this study, is important. Because we included gender as a covariate in the analyses, we expect that our group-interaction effects cannot be attributed to gender.
This is the first study to identify trait-dependent compensatory hyperactivity in the presupplementary motor area during response inhibition in both medication-free OCD patients and their unaffected siblings, possibly related to inefficiencies in neural processing within the presupplementary motor area itself. State-dependent effects on the recruitment of the right inferior parietal cortex and inferior frontal gyrus during inhibition might further contribute to the inhibition deficit in OCD. These results support the notion that impaired response inhibition is a candidate endophenotype of OCD and localize its associated neural correlate in the presupplementary motor area.