Freud first suggested that posttraumatic nightmares are compulsions to master the anxiety and guilt associated with a traumatic experience (
1). Subsequent theorists, e.g., Hanlon (
2) and Kavaler (
3), shared Freud's central notion that chronic posttraumatic nightmares represent an effort to assimilate a traumatic experience into one's psyche during sleep, when usual defense mechanisms are absent or weakened. Although psychodynamic theories of posttraumatic nightmares have been replaced by cognitively and physiologically oriented hypotheses, more recent hypotheses continue to consider chronic posttraumatic nightmares as failed attempts to adapt to and recover from traumatic experiences (
4–
6). They generally posit that sleep plays a central role in both affective and memory processes that promote either resilience or recovery from challenging waking experiences.
The discovery of rapid eye movement (REM) sleep (
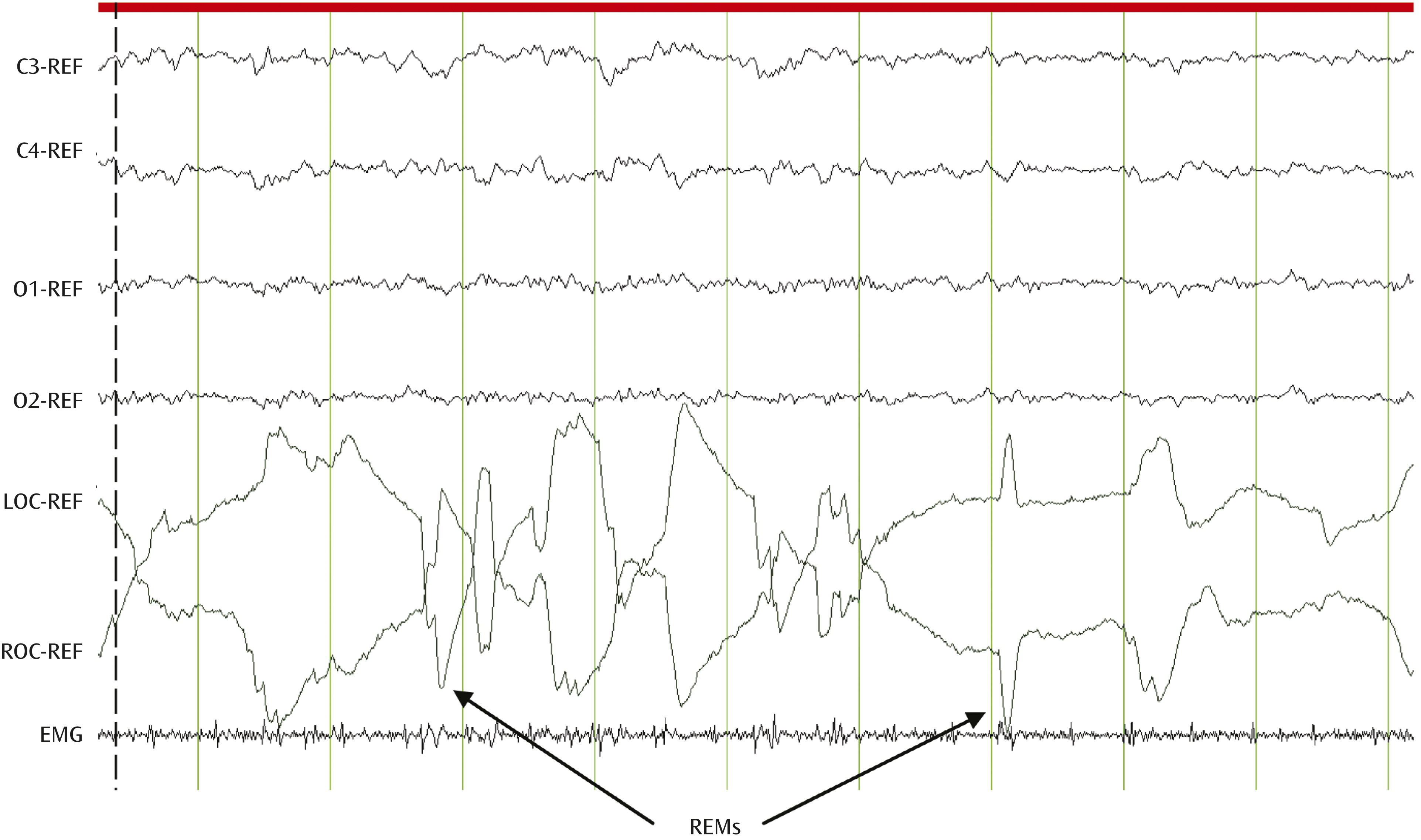
7) offered a unique window into the potential physiological and neural mechanisms subserving brain-mind interactions. REM sleep is a dynamic physiological state in which bursts of rapid eye movements (REMs) are concomitant with low-amplitude, fast-frequency electroencephalographic (EEG) activity similar to levels observed during wakefulness in the presence of muscle atonia (
Figure 1).
Increased sympathetic dominance and vagal withdrawal, cardiorespiratory irregularities, and the cessation of active thermoregulation also characterize REM sleep (
8). REM sleep recurs cyclically through the sleep period, in alternation with non-REM (NREM) sleep (
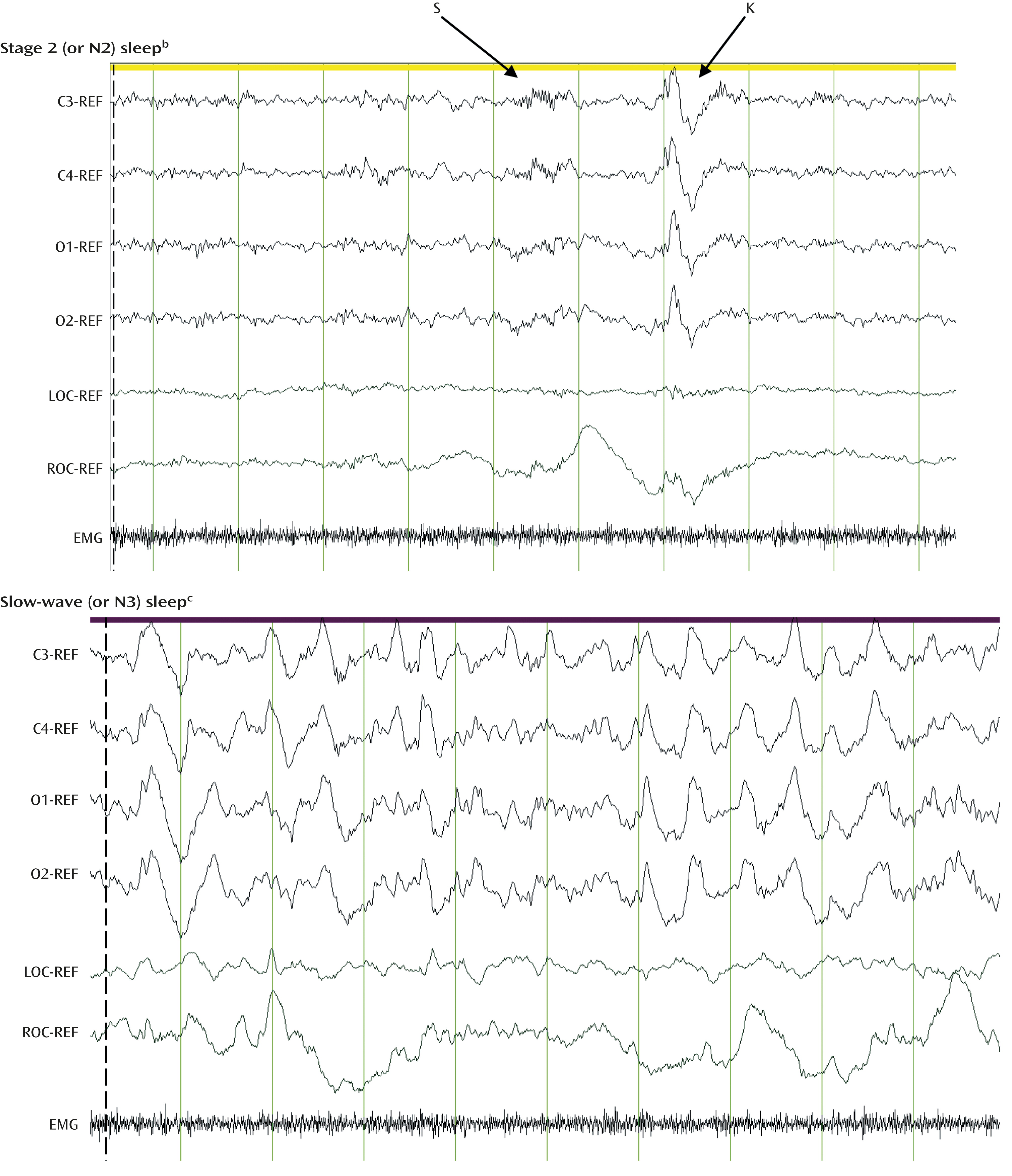
Figure 2). NREM sleep is a state of decreased arousal and overall reduction of neural activity relative to wakefulness and REM sleep.
NREM sleep is composed of both lighter sleep (N1 and N2 sleep stages; Figure 2, top) and slow-wave sleep (N3 sleep stage; Figure 2, bottom), which is conceptualized as a restorative neural state.REM sleep is regulated primarily by cholinergic cells of the pedunculopontine and laterodorsal pontine nuclei and modulated by inhibitory reciprocal interactions with noradrenergic, serotonergic, and histaminergic cells of the locus coeruleus, raphe, and tuberomammillary nuclei, respectively. In contrast to wakefulness and NREM sleep, REM sleep is characterized by the absence of noradrenergic and serotonergic activity and represents a state of heightened cholinergic activity.
While dream recall can be elicited from NREM sleep awakenings, dreaming is more prominent, vivid, and detailed during REM sleep. Nightmares are conceptualized as a phenomenon of REM sleep (
9). However, dreaming is independent from the integrity of REM-regulating pontine regions. Lesions to associative visual or occipitotemporal cortices or to the mesocortical and mesolimbic tracts in humans have been found to be associated with dreaming cessation or with vivid dreaming and altered distinction between dreaming and reality (
10).
Freud’s original suggestion that nightmares and sleep are involved in the adaptation to and recovery from trauma exposure and the discovery of REM sleep and the prominence of dreaming during this sleep state provided the foundation for the hypothesis, proposed in 1989 by Ross and colleagues (
11), that sleep disturbances, with an emphasis on REM sleep disturbances and nightmares, constitute “the hallmark of posttraumatic stress disorder (PTSD).” This hypothesis was based on the observations that 1) nightmares are a unique and core symptom of PTSD, 2) nightmares usually occur in REM sleep, 3) REM sleep is a state of increased arousal, and 4) prior laboratory-based studies have shown signs of heightened or attenuated REM sleep in PTSD, such as a greater number of REMs per REM period (an index known as REM density), higher percentage of REM sleep, and shorter latency preceding REM sleep in relation to values for comparison groups.
In the present review I aim to revisit the hypothesis that sleep disturbances are the hallmark of PTSD in light of findings derived from animal, preclinical, and clinical studies that have accumulated since 1989. After reviewing findings from clinical sleep studies in adults with PTSD, I will discuss observations derived from animal and experimental human studies focused on sleep-dependent emotion and memory processes. A more general assessment of how the literature to date coalesces to suggest that sleep disturbances may reflect a broad index of maladaptive stress responses in the face of adversity and trauma exposure will then be provided. The review will conclude with clinical implications and potential future research directions.
REM Sleep Disturbances in PTSD: Hallmark or Not?
Nightmares are primarily a REM sleep phenomenon, but they may also occur during NREM sleep in patients with PTSD (
12). These dysphoric dreams often depict themes, images, and emotions that can be related to traumatic events. Nightmares may trigger short or prolonged awakenings from sleep. However, not all distressed awakenings are associated with nightmare recalls.
Other sleep complaints reported by adults with PTSD are also more likely to arise from REM sleep. These include bad dreams unrelated to traumatic events, disruptive behaviors (such as acting-out dreams), and sleep-disordered breathing (see, for instance, references 13 and 14). Insomnia is one of the most commonly endorsed PTSD symptoms and is thought to arise primarily from NREM sleep disruption. Other NREM sleep disturbances often observed in trauma-exposed individuals and patients with PTSD include nocturnal panic attacks and sleep terrors (
13,
15). Thus, clinical observations focusing on the nature of sleep complaints in PTSD point to a dysregulation in both REM and NREM sleep.
Findings from polysomnographic studies support the clinical reports. Polysomnography refers to the collection of physiological EEG, electromyographic (EMG), and electro-oculographic (EOG) signals used for sleep staging and is the gold standard for sleep measurement. Polysomnographic studies conducted in adults with and without PTSD have yielded inconsistent findings regarding the presence or nature of REM sleep disturbances in individuals with PTSD. Some studies have reported indices of REM sleep attenuation and disruption (
16–
18), whereas other studies have shown REM sleep changes consistent with an intensification of REM sleep (
19–
22) in PTSD when compared with other clinical groups or with good sleepers without PTSD. NREM sleep disturbances have also been reported (
23–
25), but other studies have shown no detectable differences (
19,
26,
27). A meta-analysis of polysomnographic studies conducted with military veterans and civilian adults with PTSD found modest indices of objective sleep disruption in PTSD (
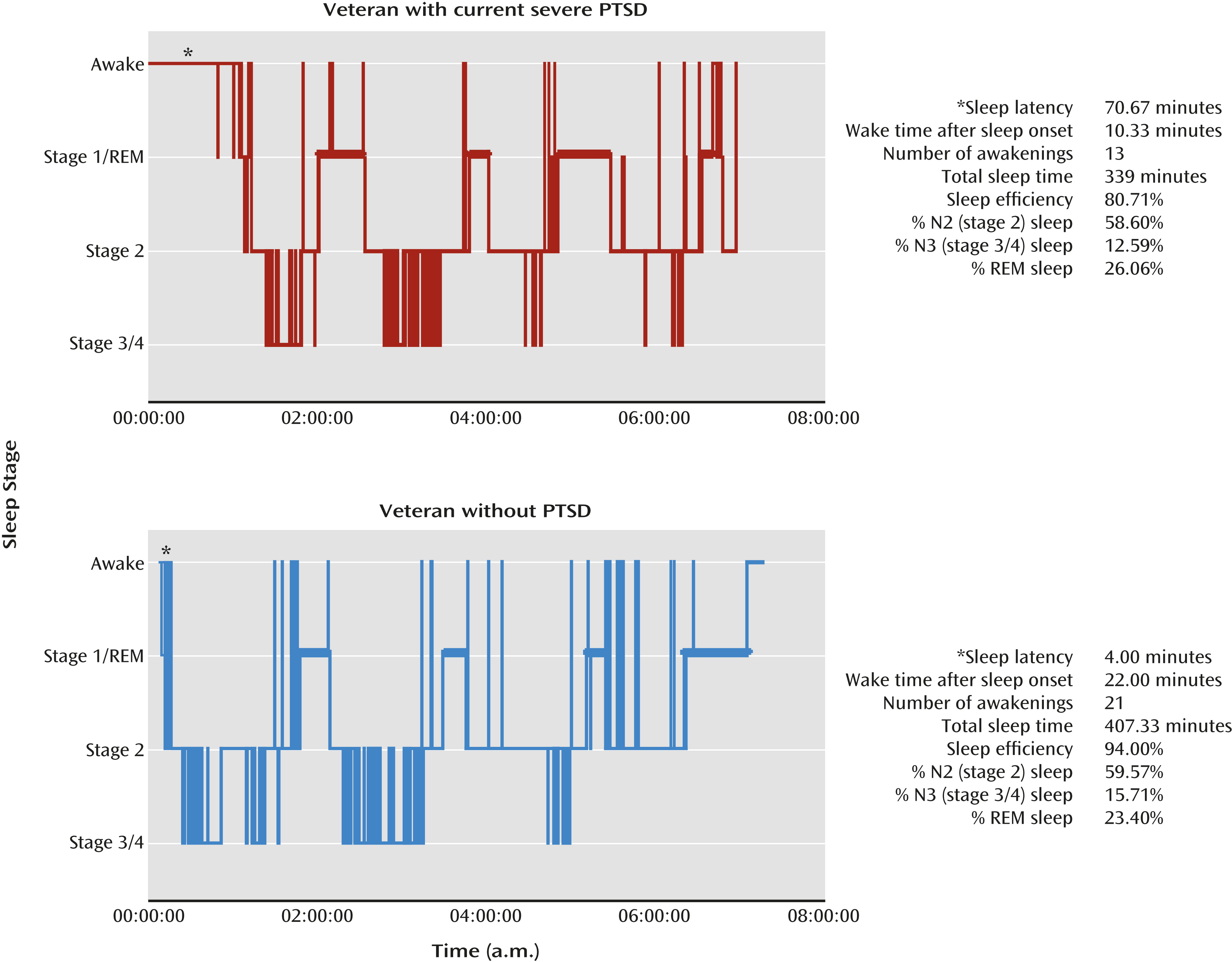
28), as indicated by more stage 1 (light) sleep (weighted effect size d
+=0.24, 95% confidence interval [CI]: 0.02 to 0.46), less slow-wave sleep (weighted effect size d
+=–0.28, 95% CI: –0.47 to –0.09]), and greater REM density than in subjects without PTSD (weighted effect size d
+=0.43, 95% CI: 0.13 to 0.73). Examples of these findings are depicted in
Figure 3.
Some of the polysomnographic differences observed in adults with PTSD overlap with those reported in adults with major depressive disorder, and no unique objective sleep profile has yet been identified when comparing different psychiatric disorders (
29).
Other objective sleep measurement methods have been used in PTSD studies. Quantitative EEG (qEEG) refers to the spectral analysis of EEG signals. qEEG-derived slow-wave activity (0.5–4.0 Hz) is used as an index of sleep pressure or sleep depth (
30,
31), whereas high-frequency beta activity (16–32 Hz) and gamma activity (32–50 Hz) are considered to be indices of central arousal during sleep (
32,
33). Some studies have shown greater than normal beta activity during NREM sleep in PTSD (
34,
35). Others, however, have indicated less beta activity during REM sleep in adults with PTSD relative to comparison subjects (
36,
37). Like polysomnography, qEEG may not be adequate to capture indices of changes in subcortical circuits during REM sleep and NREM sleep that may contribute to PTSD reliably.
Sleep neuroimaging methods have identified neurobiological differences in REM and NREM sleep between adults with depression, primary insomnia, or PTSD and healthy good sleepers, even in the absence of group differences on polysomnographic or qEEG measures (
38–
41). In a preliminary study, my colleagues and I recently observed that the increase from wakefulness to REM sleep in regional brain glucose metabolism in limbic and paralimbic regions was greater in adults with PTSD than in adults with major depression, whereas depression was associated with state-independent hypermetabolism in both wakefulness and REM sleep in these brain areas, relative to the patients with PTSD (
42). Replication in larger groups and direct comparisons among diagnostic groups are needed in order to clarify the specificity of neurobiological changes observed in REM sleep relative to wakefulness in PTSD. Comparisons of the neural correlates of NREM sleep across psychiatric diagnoses are not yet available.
In sum, findings on the nature and magnitude of polysomnographic and qEEG indices of REM sleep disturbances in PTSD are equivocal. Both subjective and objective REM and NREM sleep disturbances have been reported and may be the product of a common underlying disruption of sleep regulatory mechanisms. Whether the heterogeneity of PTSD symptoms contributes to distinct sleep profiles remains unknown. Sleep neuroimaging methods are well suited to detect neurobiological changes across sleep/wake states in PTSD or indices specifically related to nightmares, insomnia, or other disruptive nocturnal behaviors that are common in PTSD. REM sleep provides an endogenous milieu of heightened limbic activation, and NREM sleep is an endogenous milieu of attenuated arousal. Thus, both states provide unique biological states to study the potential mechanisms that contribute to sleep complaints, the pathophysiology of PTSD, or resilience.
The physiological or neural correlates of nightmares in PTSD remain largely unexplored, mainly because nightmares are rare under laboratory conditions. A comparison of objective sleep measures in adults with PTSD-related nightmares and in adults with idiopathic nightmares (i.e., nightmares unrelated to concurrent psychiatric or medical conditions without an identifiable trauma-related onset) did not show REM-specific differences between the two groups or between these groups and good sleepers (
23). Rather, adults with PTSD-related nightmares showed a greater duration of nocturnal awakenings than the other two groups. Additionally, both groups of nightmare sufferers showed more motor activity during REM and NREM sleep than did comparison subjects (
23). Nielsen and colleagues used REM sleep deprivation to unmask potential REM sleep indices that may characterize adults with idiopathic nightmares. Instead of the expected higher values on REM sleep measures indicative of a heightened propensity for REM sleep, adults with idiopathic nightmares had a lower propensity for REM sleep than good sleepers before deprivation (
43), a blunted response to REM sleep deprivation (
43), and slightly greater sympathovagal balance during REM sleep than the good sleepers after REM sleep deprivation (
44). These observations suggest that idiopathic nightmares may relate to altered REM sleep physiology. However, the cross-sectional nature of these polysomnographic studies and the absence of nightmare episodes in the laboratory make it difficult to determine the direction of the relationship between nightmares and differences in REM propensity and autonomic balance or the effects of frequent nightmares, mild levels of PTSD symptoms, and/or unspecified history of trauma exposure. Nevertheless, the REM deprivation paradigm offers a method to probe REM sleep (and potentially elicit nightmares under laboratory conditions) and to more closely evaluate the relationships between nightmares and REM sleep measures in PTSD.
Prospective Sleep Studies and Sleep Treatment Trials in PTSD
Although objective sleep findings in PTSD are mixed, there is some evidence that REM sleep disturbances are a threat to psychological resilience. REM sleep fragmentation, REM sleep autonomic imbalance, and nightmares measured within weeks following exposure to traumatic events have been associated with an increased risk for the development and persistence of PTSD (
36,
45,
46). Polysomnographic studies are costly and often impractical in large study groups. Consequently, several studies have used self-report measures of sleep disturbances in order to explore the relationships among trauma exposure, sleep complaints, and psychiatric outcomes. To date, published studies have consistently demonstrated that poor sleep and nightmares occurring soon after trauma exposure predict the onset and persistence of PTSD and other stress-related disorders, including other anxiety disorders, major depression, and addictive disorders (
47–
51). Similarly, preexisting complaints of poor sleep increase the risk of PTSD and other stress-related psychiatric disorders following trauma exposure (
52). Although these observations provide limited information regarding the possible neurobiological underpinnings of the sleep complaints, they all strongly suggest that sleep disturbances are an indicator of heightened risk for poor psychiatric outcomes following trauma exposure. More important, and unlike other risk factors for poor psychiatric outcomes (e.g., age, sex, prior trauma, and psychiatric history), sleep disturbance can be modified, through sleep-focused treatments.
Indeed, a number of sleep treatment trials have shown that improvements in nightmares and insomnia following targeted treatments are accompanied by improvements in the severity of daytime symptoms of PTSD, depression, and anxiety. Subjective sleep complaints can be reduced with recommended PTSD treatments (
53), but nightmares and insomnia are often treatment resistant. A number of pharmacological and behavioral treatments targeting sleep disturbances in PTSD have been used (see reference
54 for review; see also reference
55). To date, prazosin (an alpha-1 antagonist) (
56,
57) and imagery rehearsal therapy and its variants (
58,
59) are the recommended treatment options for PTSD-related nightmares and are associated with improvements in nightmares and insomnia (
60), although there have also been negative results (
61). Combined behavioral treatments for nightmares and insomnia applied to PTSD patients have also yielded promising findings (
62,
63). Overall, treatment studies suggest that the normalization of sleep disturbances has beneficial effects on other disrupted neurobiological mechanisms that contribute to daytime PTSD symptoms.
Only a few clinical trials have combined polysomnography and other objective sleep measurement methods to evaluate whether sleep treatments are associated with detectable improvements in sleep. One study found that prazosin was associated with greater total sleep time, greater REM sleep duration, and shorter REM latency than placebo in a crossover trial with adult civilians who had chronic PTSD and psychiatric comorbidities (
64). Thus, REM sleep disruption induced by hypernoradrenergic activity during sleep may be normalized by noradrenergic blockade with prazosin, which facilitated REM sleep consolidation and may mediate improvements in sleep complaints. Changes in REM sleep were not observed after an 8-week double-blind randomized trial that compared prazosin, placebo, and a behavioral sleep intervention targeting nightmares and insomnia in a group of 50 military veterans with symptoms of posttraumatic stress (
63). The same was true after a 12-week open-label trial with nefazodone in 12 military veterans with chronic PTSD (
65). Modest increases in the percentage of REM sleep and REM density concurrent with a reduction in REM sleep latency (together suggesting increased REM sleep pressure) have been reported following imagery rehearsal therapy (
66). The changes in REM sleep were paralleled by improvements in nightmares, sleep complaints, and daytime symptoms of PTSD, anxiety, and depression. The effects of sleep treatments on the neurobiology of PTSD during wakefulness and REM remain unknown.
Sleep-Dependent Emotion and Memory Processes: Animal and Human Studies
The relationships of sleep to learning and memory (
67,
68), emotional processing (
69,
70), and adaptation to stress (
71–
73) in animals and humans have long been recognized. These have provided further support to the hypothesis that sleep disturbances may contribute to the pathophysiology of PTSD.
Classical fear conditioning and extinction paradigms have been used in both animal and human studies in order to evaluate the role of sleep (and sleep disturbances) in fear responses as an experimental model of PTSD. Classical (or Pavlovian) cued or contextual fear conditioning arises when a neutral stimulus (e.g., light, tone, specific environment) closely precedes in time the occurrence of an aversive, emotionally significant event (e.g., shock) that elicits a fear response (e.g., freezing, increased skin conductance response). The neutral stimulus or environment is referred to as the “conditioned stimulus,” and the aversive event is referred to as the “unconditioned stimulus.” With repetition of the association, the conditioned stimulus alone can elicit the fear response, now called the “conditioned response.” Fear extinction refers to a competing learning process that parallels the attenuation and loss of the conditioned response following repeated presentations of the conditioned stimulus alone. Contextual fear conditioning follows a similar paradigm, except that the context in which the unconditioned stimulus is presented is used as the conditioned stimulus.
In rodents, fear conditioning decreases REM sleep latency, decreases REM sleep duration and number of REM bouts, and increases ponto-geniculo-occipital waves (
74–
76). Alternatively, REM sleep is increased following safety conditioning (where animals learn that they will not be exposed to aversive stimuli in a given environment or given a specific cue never paired with the aversive stimulus) (
76) or shuttle-box avoidance training (where the animals learn that they can terminate the unconditioned stimulus by escaping to a different compartment of the training cage (
67–
77). Depriving rats of REM sleep before conditioning impairs both cued and contextual fear conditioning, as well as learning and recall on a discriminative avoidance task (
78,
79). Alternatively, postconditioning REM sleep deprivation impairs extinction of cued but not contextual fear conditioning (
80), and extinction of contextual conditioned fear is associated with increased sleep (
81). Thus, animal studies suggest that fear responses affect and are affected by REM sleep, as well as indicating a more general relationship between sleep and memory processes.
Currently, there is no animal model in which to study the effects of nightmares or re-exposure to the conditioned stimulus during sleep. Such models are necessary in order to unravel the possible distinct and additive effects of REM sleep abnormalities and nightmares on the onset and persistence of PTSD. Of course, these models will require the use of physiological outcome measures analogous to those captured in humans (e.g., blood pressure, heart rate) to assess the magnitude of fear responses upon representation of the conditioned stimulus during REM (or NREM) sleep, as the behavioral measure of freezing has obvious limitations in the sleeping animal.
Fear conditioning, extinction, and extinction recall have been studied in humans, and the results suggest that fear responses can affect and be affected by REM sleep. In one study, overnight sleep following fear conditioning and extinction facilitated generalization of extinction to unextinguished stimuli more than did a comparable period of wakefulness (
82). It remains unknown, however, whether REM sleep mediated this effect. Subjects who exhibited REM sleep during a nap following fear conditioning and extinction showed lower skin conductance response and greater activation of the ventromedial prefrontal cortex in response to the extinguished stimulus when compared with subjects who did not achieve REM sleep during the nap (
83). Additionally, those who exhibited REM sleep during their nap also had lower skin conductance response and activation of neural activity in the laterodorsal tegmentum in response to electrical shocks during conditioning. These findings suggest that individual differences in REM sleep propensity modulate both fear conditioning and extinction recall.
Human studies that have probed the role of sleep in affective and memory processes further support the hypothesis that sleep disturbances may contribute to the pathophysiology of maladaptive stress responses, including PTSD. Extensive and elegant reviews of this literature are available elsewhere (
84–
86).
Work by Walker and colleagues highlighted the relevance of these studies in understanding the potential role of sleep, and more specifically REM sleep, in PTSD. Specifically, their studies revealed that overnight sleep deprivation enhanced neural response to negative emotional stimuli, by simultaneously potentiating amygdala activation and reducing activation of the medial prefrontal cortex in response to negative stimuli. Furthermore, sleep deprivation reduced the connectivity between the amygdala and medial prefrontal cortex (87). In another study, they showed that napping after an emotion task reduced reactivity to anger and fear-related stimuli and increased reactivity to positive stimuli (88). It is possible that these effects may be mediated by REM sleep during naps. REM sleep during naps has also been associated with increased emotional memory consolidation (
86). Together, these findings reinforce the hypothesis that sleep disturbances play an important role in PTSD by compromising sleep-dependent affective and memory processing. Similar experimental paradigms applied to trauma-exposed individuals with and without PTSD could clarify the relationship of sleep or REM sleep to neural responses to trauma-relevant or general emotional cues. Such findings may inform how sleep (or napping) may be scheduled in relation to cognitive-behavioral treatment sessions to enhance therapeutic effects.
Sleep Disturbances: A Marker of Compromised Psychological Resilience?
Disturbed sleep may constitute a risk factor for poor psychiatric outcomes following trauma exposure, as suggested by the aforementioned observation that survivors of motor vehicle accidents who showed autonomic and polysomnographic REM sleep disturbances were at a higher risk of developing PTSD over time (
36,
45). Additionally, preexisting and posttrauma sleep complaints increased the risk for PTSD and other stress-related disorders (
52).
There are other indications that sleep disturbances, including REM sleep disruption, may indeed be sensitive indices of compromised resilience. Individual differences in REM sleep propensity have been observed in both animals and humans, and they moderate the effects of stress and/or trauma exposure on behavioral and physiological outcomes (
83,
89). Furthermore, REM sleep is most prominent prenatally and over the first 2 years of life. Challenges or significant adversity during this developmental period may have a more significant impact on the physiology of REM sleep. Exposure to acute or chronic stressors or trauma during childhood or adolescence can affect brain development (
90) and REM sleep physiology. Consistent with this suggestion are findings indicating that childhood adversity is strongly associated with adult sleep complaints (
91,
92) and is prevalent among adults with insomnia who also show elevated indices of objective sleep disruption (
93). Additionally, REM sleep fragmentation in adult combat-exposed military veterans was strongly associated with exposure to adversity before age 18, as well as with adult self-reported disruptive nocturnal behaviors (
94). These findings raise the possibility that the robust predictive relationship between insomnia and an increased risk for subsequent mood, anxiety disorders, and addictive disorders (
51,
95) may be closely related to early exposure to adversity.
To date, studies focused on sleep in PTSD have all been conducted with adults exposed to trauma or with chronic PTSD. Whether objective sleep disturbances precede trauma exposure and/or the onset of PTSD and whether the nature of sleep disturbance changes over time remain undetermined. However, there is rapidly growing evidence that individual difference in sleep or in REM sleep may be a marker of vulnerability to poor psychiatric outcomes following trauma exposure or, alternatively, of resilience.
Summary
Since Ross and colleagues proposed the hypothesis that sleep disturbances are the hallmark of PTSD and emphasized REM sleep disturbances, several human and animal studies have provided evidence supporting a role for sleep in PTSD-relevant emotion and memory processing, even in the absence of robust objective profiles indicative of REM sleep disruption. Prospective follow-ups of trauma-exposed individuals and sleep treatment studies reinforce the relationship between sleep disruption and poor psychiatric outcomes and between sleep restoration and improvements in daytime symptoms. However, the biological mechanisms underlying these relationships remain unknown. There is evidence that individual differences in REM sleep may mediate the effects of stress or trauma on behavioral, physiological, and neural responses to stressors in both animals and humans. These effects, however, may not be limited to REM sleep alone. Rather, both animal and human studies provide strong support for the notion that sleep, more generally, is directly involved in PTSD-relevant emotion and memory processes. These findings have important clinical implications and provide directions for future research.
Clinical Implications
The finding that sleep disturbances preceding or following traumatic experiences contribute to poor psychiatric outcomes offers new strategies for prevention and early detection efforts in high-risk populations and for individuals exposed to traumatic events. Specifically, regular sleep assessments in high-risk populations and in trauma-exposed individuals may facilitate the provision of management strategies aimed to enhance resilience through the delivery of sleep-promoting habits. Unless sleep-disordered breathing is suspected, ambulatory or laboratory-based sleep studies are not necessary for the evaluation and treatment of nightmares, insomnia, or other complaints of poor sleep. However, clinical interviews, validated self-report measures, and/or sleep diaries can effectively guide the diagnostic process and development of comprehensive treatment plans that address trauma-related sleep complaints. Early delivery of effective pharmacological and behavioral treatments to enhance sleep consolidation and/or to accelerate the restoration of sleep in the early aftermath following trauma exposure may ultimately accelerate recovery.
Currently, there is no consensus or guideline regarding the inclusion of evidence-based sleep treatment strategies in the context of trauma and PTSD management, nor is it the case in the context of conditions frequently comorbid with PTSD, such as depression, suicidality, and addictive disorders. In addition, there are no empirical data available at this time to determine the order in which PTSD and sleep treatments should be delivered. Until ongoing studies clarify this question, clinicians need to consider that the chronic sleep disruption associated with nightmares and insomnia may affect the efficacy of first-line PTSD treatments. This is especially the case for cognitive-behavioral approaches that rely on habituation (or reduction of reactivity to fear-provoking stimuli and situations) or on cognitive processing, which are two brain functions markedly affected by sleep loss.
Finally, there is a need to facilitate education about effective pharmacological and behavioral sleep treatments for mental health care providers involved in the treatment and management of patients suffering from PTSD. Conversely, there is a considerable need to educate sleep clinicians on the potential effects of trauma exposure on sleep.
Future Directions for Sleep Studies of PTSD and Other Stress-Related Psychiatric Disorders
Since 1989, there has been a growing interest in understanding how sleep contributes to the interactions among physiological, neural, and psychological mechanisms that underlie the pathophysiology of PTSD. The vast majority of the work to date has been cross-sectional, with the exception of a few small prospective studies in trauma-exposed study groups and treatment studies. A minority of the latter have included objective sleep measurement methods that may provide insights into the physiological or neural underpinnings of the relationships of sleep to clinical gains and PTSD symptoms. The field is ripe for prospective and longitudinal studies in high-risk groups in order to understand how changes in REM sleep (or more generally, sleep physiology and neurobiology) contribute to increased risk of poor psychiatric outcomes.
Much of the previous work on sleep and PTSD has also been limited to adults, often with chronic PTSD and high rates of psychiatric comorbidities and use of psychotropics. Findings from animal and human studies converge to suggest that early adversity during critical developmental periods can have long-lasting effects on REM sleep and brain development (
96). Assessing the impact of stress and trauma on sleep in children and adolescents and their relationships to psychiatric outcomes later in life may yield significant insights into pathways to prevention.
Animal models that have been used as analogues of human PTSD have provided information on the effects of trauma exposure on sleep and the effects of pre- or postconditioning sleep deprivation on subsequent fear responses. Human studies have used total sleep deprivation or REM sleep deprivation as probes, but these models have not yet been applied to individuals with PTSD. Similarly, studies of sleep-dependent emotion and memory processes have yielded highly relevant findings for PTSD, but these methods have not been applied to trauma-exposed groups with or without PTSD. Extensions of these findings to clinical groups are awaited. The combination of high-density EEG and other approaches to assess brain connectivity in these individuals have the potential of clarifying the direction of the relationship between fear responses and REM sleep. Finally, translational efforts are needed to accelerate the identification of risk and protective factors and the development of novel treatment targets to accelerate normalization of trauma reactions, as well as recovery from PTSD.
In conclusion, the hypothesis that sleep disturbances are the hallmark of PTSD has stimulated a wealth of animal and human studies to clarify and examine the role of sleep and sleep disturbances in stress responses. Data accumulated to date suggest that sleep disturbances are the hallmark not only of PTSD but also of a heightened vulnerability to maladaptive stress responses. Disturbed sleep is a modifiable risk factor, and sleep restoration through effective targeted sleep treatments may accelerate recovery from trauma exposure and PTSD. Identifying the relationships between sleep and affective and memory processes and clarifying the neural underpinnings of these relationships can provide novel directions for the development of innovative strategies for risk management and treatment.
Acknowledgments
The author thanks Jessica Boarts, Ph.D., and 2nd Lt. Benjamin Paul for editorial comments.