In this issue of the
Journal, Vizueta et al. (
1), a research group at the University of California, Los Angeles, led by Lori Altshuler, M.D., present findings from their latest investigation into the neural correlates of mood states in bipolar disorders. Specifically, they provide novel evidence for relatively decreased corticolimbic reactivity and functional connectivity during the perceptual processing of affectively charged stimuli in patients with bipolar II disorder. In and of itself, the study usefully informs our understanding of potential pathophysiologic neural mechanisms in bipolar II disorder, which has received less attention than bipolar I disorder. However, the greater reach of this report is found within the broader context of the investigators' ongoing research seeking to identify the neural correlates of cyclic pathologic mood states as characterized by bipolar disorders.
In the June 2005 issue, Altshuler et al. (
2) presented the initial results from this line of research. In that seminal report, the authors discovered relatively increased amygdala reactivity in patients with bipolar I disorder during a manic episode. This pattern is consistent with the role of the amygdala in mediating increases in not only physiologic but also behavioral arousal in response to environmental stimuli. This is accomplished by modulating neuronal populations in the basal forebrain providing cholinergic tone to the neocortex as well as neuromodulatory centers in the brainstem controlling distributed monoaminergic signaling (
3). In many ways, such amygdala hyperreactivity in bipolar disorder is consistent with the euphoria, irritability, insomnia, inattention, and distractibility that often define a manic episode. Subsequent to this study of manic bipolar I patients, the research team looked for contrasting decreases in amygdala reactivity in depressed bipolar I patients, who typically exhibit relatively low physiologic and behavioral arousal.
Consistent with their expectations, Altshuler et al. observed relatively decreased amygdala reactivity in depressed bipolar I patients relative to healthy comparison subjects (
4). However, this difference failed to reach statistical significance in formal tests. In contrast, statistically significant differences between depressed bipolar I patients and comparison subjects emerged in several prefrontal regions, including decreased activity in lateral aspects of the orbitofrontal cortex (BA 47, also referred to as the ventrolateral prefrontal cortex) and the dorsolateral prefrontal cortex (BA 9) but relatively increased activity in the frontopolar cortex (BA 10). Interestingly, the same BA 47 regions of the orbitofrontal cortex also exhibited a relatively attenuated response during the earlier study of manic bipolar I patients. Moreover, a similar attenuated response in orbitofrontal cortex BA 47 has been reported by this same group in euthymic bipolar I patients (
5). Notably, in that study, amygdala reactivity did not significantly differ between euthymic bipolar I patients and healthy comparison subjects. Now, the team again reports relatively decreased activity in orbitofrontal cortex BA 47 in depressed bipolar II patients.
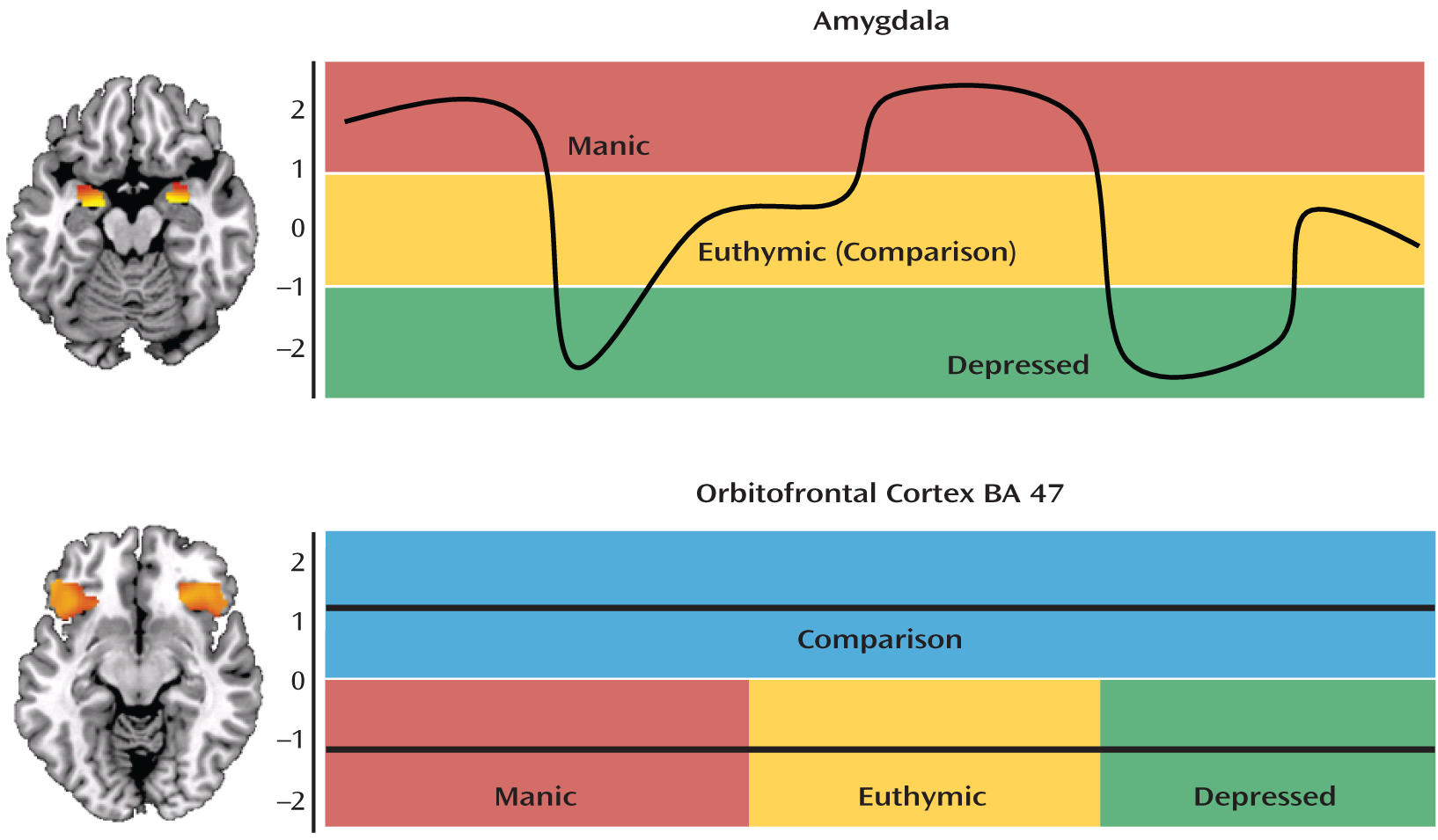
When we look across these prior studies of bipolar I disorder and the Vizueta et al. findings on depressed bipolar II patients, a striking pattern emerges: amygdala reactivity follows a state-dependent course (i.e., increased in manic, decreased in depressed, and unchanged in euthymic states), while prefrontal activation exhibits a state-independent or trait-like course (i.e., decreased in all three states) (
Figure 1). Critically, the BA 47 regions of the orbitofrontal cortex exhibiting trait-like hypoactivation across manic, depressed, and euthymic states play an important role in the down-regulation of amygdala reactivity (
6–
8). In healthy volunteers, BA 47 appears to exert top-down regulatory control over the amygdala (likely through medial prefrontal regions with direct amygdala connections), as indexed by negative functional connectivity between the two regions. Balance in this dynamic functional circuitry is critical for the expression of behavioral and physiologic responses to provocation that are both temporally limited and contextually appropriate (
9). The data accrued by Altshuler et al. suggest that during mania, the BA 47 regions of the orbitofrontal cortex may not be adequately capable of regulating amygdala reactivity. In fact, in a follow-up study of their original report on manic bipolar I patients, the research team demonstrated decreased negative functional connectivity between orbitofrontal cortex BA 47 and the amygdala in patients relative to healthy comparison subjects (
10).
If amygdala reactivity is a state-dependent phenomenon in bipolar disorders and trait-like hypoactivation of regulatory orbitofrontal regions creates a permissive environment for the translation of amygdala hyperreactivity into manic symptoms, then what could be driving the amygdala hyporeactivity in the depressed state reported in the Vizueta et al. study of bipolar II patients and suggested by the research team's earlier study of bipolar I patients? Again, the relative functioning of prefrontal regions may be key. In the present study of depressed bipolar II patients, Vizueta et al. do not report decreased negative functional connectivity between the amygdala and orbitofrontal cortex BA 47, which can be interpreted as less top-down regulation, as observed in manic bipolar I patients, but rather they report increased positive functional connectivity between these two regions. A similar increase in positive functional connectivity between the amygdala and orbitofrontal cortex BA 47 has been reported by Versace et al. in depressed bipolar I patients (
11). Thus, it is possible that the depressed state of bipolar disorders reflects dysfunctional positive connectivity between these two nodes, resulting in inappropriate inhibition of the amygdala. Unfortunately, measures of functional connectivity from blood-oxygen-level-dependent functional MRI data cannot decipher such interactions as being either excitatory or inhibitory in nature. Regardless, these data suggest that it is not simply differences in the activation of brain regions (i.e., orbitofrontal cortex BA 47 is hypoactive across states) but rather alterations in the functional connectivity of distributed regions (i.e., decreased amygdala-orbitofrontal cortex BA 47 connectivity in mania but increased connectivity in depression) that may be particularly important for understanding changes in mood states.
Before one gets too excited about the potential of these data to illuminate a mechanism for cyclic pathologic mood states in bipolar disorders, it is important to note that the literature, particularly with regard to depressed bipolar I disorder, is decidedly mixed at the moment, with some studies finding increased, others decreased, and yet others no significant change in amygdala reactivity (
12). There are many likely contributors to this heterogeneity, including studies that 1) collapse patients in manic, depressed, and euthymic states, 2) focus on medicated or nonmedicated patients, and 3) use a variety of experimental paradigms. A notable methodological strength of the research conducted by Altshuler's team is the use of the same functional task paradigm in all of their studies, which has facilitated cross-sectional comparisons across mood states in bipolar I and II disorders. Longitudinal research within a cohort of patients is now necessary to identify the neural basis of the transition between states and the triggers for these transitions. In this context, it will be interesting to establish how observed changes in corticolimbic circuitry map onto specific dimensional aspects of dysfunction, an element missing from the current research.
Altshuler's research team, as well as other groups (
13), have begun longitudinal research of bipolar I patients as they move through manic, depressed, and euthymic episodes, and their work should provide further insight into the pathophysiology of cyclic mood states in bipolar disorders. Moreover, the existing research, as well as that under way, usefully informs and encourages a broadening of our appreciation of amygdala reactivity and functional connectivity with prefrontal regions, moving beyond simply negative emotionality to encompass complex and perhaps unexpected influences over behavioral arousal and responsiveness. Moving forward with such a more nuanced approach will hopefully yield a deepening in our understanding of mood states in both health and disease.