Nonpharmacological Interventions for ADHD: Systematic Review and Meta-Analyses of Randomized Controlled Trials of Dietary and Psychological Treatments
Abstract
Objective
Method
Results
Conclusions
Method
Inclusion Criteria
Search Strategy
Outcome Measure
Study Selection
Data Extraction
Statistical Analysis
Results
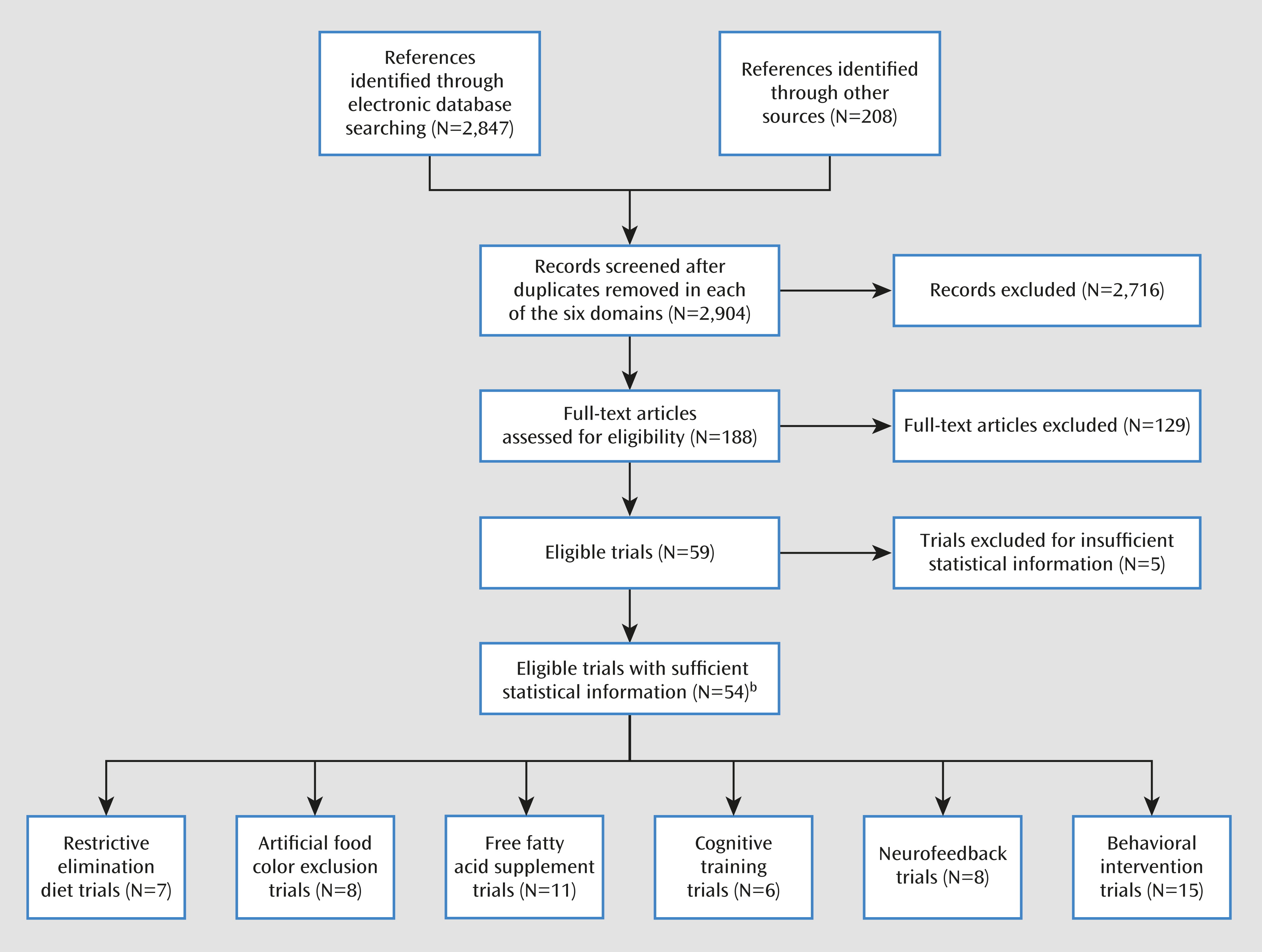
| Numbers Randomized | Characteristics | ADHD Measure | |||||||
|---|---|---|---|---|---|---|---|---|---|
| First Author (Reference) | Treatment | Control | Reported Design Qualityb | Treatment | Control | Age (Years; Mean or Range) | Male (%) | Most Proximal Assessment | Probably Blinded Assessment |
| Restricted elimination diets | |||||||||
| Pelsser (16) | Elimination diet | Waiting list | 3 | 50 | 50 | 3–9 | 86 | P-ARS | None |
| Boris (31) | Known antigenic foods | Placebo | 5 | 16 | 16 | 7.5 | 69 | CPRS | CPRS |
| Kaplan (32) | Known antigenic foods | Placebo | 3 | 25 | 25 | 3–6 | 100 | CPRS | CTRS |
| Carter (33) | Specific provoking food | Placebo | 5 | 19 | 19 | 3–12 | 74 | CPRS | Test session observation |
| Egger (34) | Specific provoking food | Placebo | 5 | 31 | 31 | 3–12 | 88 | Psychologist rating | Psychologist rating |
| Pelsser (35) | Elimination diet | Waiting list | 3 | 15 | 12 | 3–9 | 81 | CPRS | None |
| Schmidt (36) | Oligoantigenic diet | Control diet | 4 | 49 | 49 | 7–12 | 96 | CTRS | CTRS |
| Artificial food color exclusions | |||||||||
| Goyette (37)c | Certified food colors | Placebo | 1 | 17 | 17 | 4–12 | n.a. | CPRS | CTRS |
| Goyette (37)d | Certified food colors | Placebo | 1 | 13 | 13 | 3–10 | n.a. | CPRS | CTRS |
| Harley (38) | Certified food colors | Placebo | 4 | 9 | 9 | 9.2 | 100 | CPRS | CTRS |
| Williams (39) | Certified food colors | Placebo | 4 | 29 | 29 | 6–14 | 93 | CPRS | CTRS |
| Conners (40) | Kaiser Permanente diet | Control diet | 4 | 17 | 17 | 6–13 | n.a. | CPRS | CTRS |
| Harley (41) | Feingold diet | Control diet | 3 | 36 | 36 | 6–13 | 100 | CPRS | CTRS |
| Levy (42) | Tartrazine | Placebo | 3 | 8 | 8 | 5.2 | 88 | CPRS | CPRS |
| Adams (43) | Unspecified food colors | Placebo | 3 | 18 | 18 | 4–12 | 83 | Unstandardized parent rating | Unstandardized parent rating |
| Free fatty acid supplementation | |||||||||
| Bélanger (44) | Omega-3 | Placebo | 3 | 19 | 18 | 8.3 | 69 | CPRS | CPRSC |
| Gustafsson (45) | Omega-3 | Placebo | 5 | 46 | 46 | 7–12 | 80 | CPRS | CTRSD |
| Johnson (46) | Omega-3 | Placebo | 5 | 37 | 38 | 8–18 | 85 | P-ARS | P-ARS |
| Stevens (47) | Omega-3 | Placebo | 3 | 25 | 25 | 6–13 | 87 | P-CASQ | T-CASQ |
| Voigt (48) | Omega-3 | Placebo | 5 | 27 | 26 | 6–12 | 78 | CBCL (attention) | CBCL (attention) |
| Aman (49) | Omega-6 | Placebo | 4 | 31 | 31 | 8.9 | 87 | P-RBPC (attention) | CTRS |
| Arnold (50) | Omega-6 | Placebo | 4 | 18 | 18 | 6–12 | 100 | CTRS average | CTRS average |
| Hirayama (51) | Omega-3, -6 | Placebo | 4 | 20 | 20 | 6–12 | 80 | Symptom counte | Symptom counte |
| Manor (52) | Omega-3, -6 | Placebo | 5 | 137 | 63 | 6–13 | 70 | CPRS | CTRS |
| Raz (53) | Omega-3, -6 | Placebo | 4 | 39 | 39 | 7–13 | 60 | P-ARS | CTRS |
| Sinn (54) | Omega-3, -6 | Placebo | 4 | —f | —f | 7–12 | 74 | CPRS | CPRS |
| Cognitive training | |||||||||
| Rabiner (55) | Attention training | Waiting list | 2 | 25 | 25 | n.a. | 69 | CTRS (inattention) | CTRS (inattention) |
| Shalev (56) | Attention training | Computer game | 2 | 20 | 16 | 6–13 | 83 | CPRS | CPRS |
| Steiner (57) | Attention training | Waiting list | 3 | 13 | 15 | 12.4 | 52 | CPRS | CTRS |
| Johnstone (58) | Working memory training | Easy training | 3 | 20 | 20 | 8–12 | 85 | Purpose-designed rating scale, parents | Purpose-designed rating scale, parents |
| Johnstone (59) | Working memory training | Waiting list | 2 | 22 | 20 | 7–12 | 86 | Purpose-designed rating scale, parents | None |
| Klingberg (60) | Working memory training | Easy training | 5 | 26 | 27 | 7–12 | 82 | CPRS | CTRS |
| Neurofeedback | |||||||||
| Steiner (57) | Theta-beta training | Waiting list | 3 | 13 | 15 | 12.4 | 52 | CPRS | CTRS |
| Bakhshayesh (61) | Theta-beta training | EMG biofeedback | 3 | 18 | 17 | 6–14 | 74 | P-FBB-HKS | T-FBB-HKS |
| Beauregard (62) | Theta-beta training | No treatment | 1 | 15 | 5 | 8–12 | 55 | CPRS | None |
| Holtmann (63) | Theta-beta training | Cognitive exercise | 2 | 20 | 14 | 7–12 | 91 | P-FBB-HKS | None |
| Linden (64) | Theta-beta training | Waiting list | 1 | 9 | 9 | 5–15 | n.a. | P-SNAP | None |
| Heinrich (65) | Slow cortical potential training | Waiting list | 2 | 13 | 9 | 7–13 | 95 | P-FBB-HKS | None |
| Gevensleben (66) | Theta-beta and slow cortical potential training | Cognitive exercise | 2 | 64 | 38 | 8–12 | 82 | P-FBB-HKS | T-FBB-HKS |
| Lansbergen (67) | IFBT | Placebo neurofeedback | 4 | 8 | 6 | 8–15 | 93 | P-ARS | P-ARS |
| Behavioral interventions | |||||||||
| Bor (68) | Parent training | Waiting list | 2 | 26 | 37 | 3.6 | 73 | ECBI (inattention) | None |
| Hoath (69) | Parent training | Waiting list | 1 | 9 | 11 | 5–9 | 76 | P-CAPS | T-CAPS |
| Jones (70) | Parent training | Waiting list | 3 | 50 | 29 | 3.8 | 68 | CPRS | None |
| Pisterman (71) | Parent training | Waiting list | 2 | 23 | 22 | 4.1 | 91 | Home observation | Home observation |
| Sonuga-Barke (72) | Parent training | Attention control | 4 | 30 | 28 | 2–4 | 62 | PACS | Home observation |
| Sonuga-Barke (73) | Parent training | Waiting list | 4 | 59 | 30 | 2–4 | n.a. | PACS | None |
| Thompson (74) | Parent training | Waiting list | 5 | 21 | 20 | 2–6 | 73 | PACS | Home observation |
| van den Hoofdakker (75) | Parent training | Treatment as usual | 2 | 48 | 48 | 4–12 | 76 | CPRS | None |
| Evans (76) | Parent and child training | Treatment as usual | 1 | 31 | 18 | 11–13 | 71 | P-ARS | None |
| Fehlings (77) | Parent and child training | Nondirective therapy and/or support | 2 | 13 | 13 | 8–11 | 100 | P-WWAS | None |
| Horn (78) | Parent and child training | Placebo | 2 | 16 | 16 | 7–11 | n.a. | CPRS | None |
| Webster-Stratton (79) | Parent and child training | Waiting list | 3 | 49 | 50 | 6.4 | 75 | CPRS | CTRS |
| Bloomquist (80) | Child, parent, and teacher training | Waiting list | 2 | 20 | 16 | 8.5 | 69 | CTRS | None |
| MTA (81) | Child, parent, and teacher training | Treatment as usual | 3 | 144 | 146 | 8.3 | 80 | P-SNAP | Classroom observation |
| Brown (82) | Child training | Nondirective therapy and/or support | 2 | 10 | 8 | 5–13 | 85 | CPRS (hyperactivity) | ACTRS |
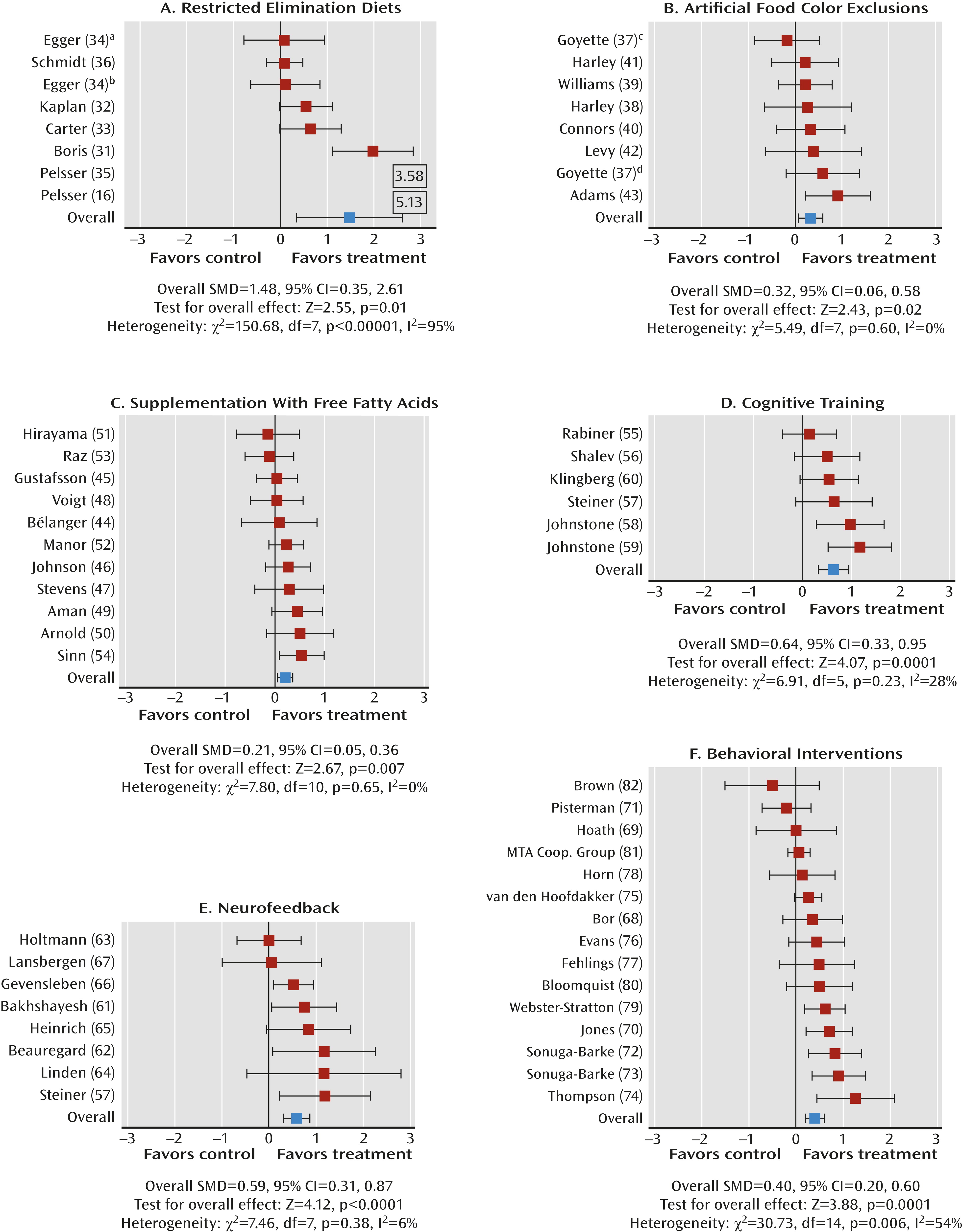
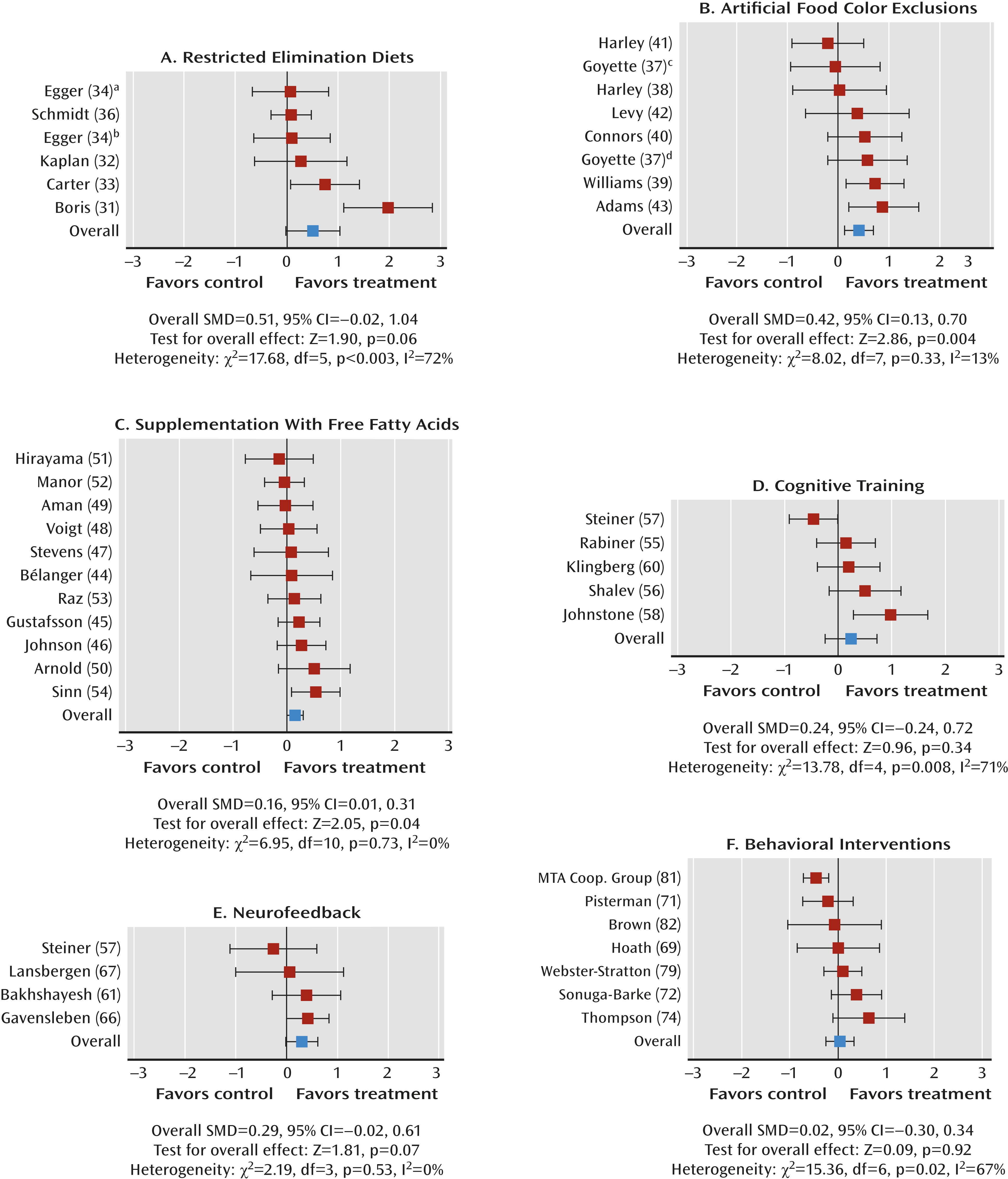
Dietary Interventions
Restricted elimination diets.
Artificial food color exclusions.
Free fatty acid supplementation.
Psychological Interventions
Cognitive training.
Neurofeedback.
Behavioral interventions.
Effect of study quality.
Discussion
Conclusions
Acknowledgments
Footnote
Supplementary Material
- View/Download
- 210.74 KB
References
Information & Authors
Information
Published In
History
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing PsychiatryOnline@psych.org or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

