In adult anxiety disorders, poor threat-safety discrimination is thought to arise from prefrontal cortex dysfunction that can be ameliorated by exposure therapy (
1). Adolescent anxiety disorders are impairing, predict high risk for adult anxiety disorders, and, like adult anxiety disorders, respond to exposure therapy (
2,
3). However, while research has identified the neural correlates of threat-safety discrimination in adult anxiety disorders (
4), there are no parallel studies in adolescents. In this study, we used conditioning, extinction, and a threat-safety discrimination paradigm to compare prefrontal cortex function in anxious and healthy adolescents and adults (
5).
Conditioning and extinction are often used in animal studies to examine fear and safety learning (
6,
7). In fear conditioning, a previously neutral conditioned stimulus (CS+) acquires threat value after it is paired with an aversive unconditioned stimulus (UCS). In extinction learning, the CS+ is presented in the absence of the aversive UCS, typically resulting in a new CS+-safe association. The level of fear experienced in response to subsequent encounters with the CS+ is thought to reflect competition between memory for the original threat and subsequent safety associations.
Cross-species work has mapped circuits supporting memory for an extinguished CS+ in the ventral prefrontal cortex (
8). When humans encounter an extinguished CS+, they engage the subgenual anterior cingulate cortex and the ventromedial prefrontal cortex, two medial prefrontal cortical subregions. Moreover, levels of activation are lower in anxious adults than in nonanxious adults (
4,
9). However, no research has examined prefrontal cortex responses to a CS+ following fear conditioning and extinction in healthy or anxious adolescents. To do so, we adapted previously used paradigms in which neutral faces served as the CS (
10,
11) and a scream served as the UCS (
12,
13). While previous functional MRI (fMRI) studies of healthy youths used our paradigm to study conditioning (
13), we instead compared neural responses to a threat following conditioning and extinction in healthy and anxious adolescents and adults.
First, we studied participants in the clinic to compare responses during fear acquisition and extinction. Based on previous research, we hypothesized that compared with healthy individuals, anxious individuals would condition similarly, measured by relative differences between the CS+ and CS– (the CS not predicting the UCS), but would report greater subjective anxiety in response to both the CS+ and CS–. We further expected both anxious groups to exhibit lower levels of extinction than the two healthy groups (
14).
Next, we used fMRI to map brain regions engaged during exposure to an array of CS+ and CS– stimuli 3 weeks after extinction. This array contained images created by morphing the previously extinguished CS+ with the CS– in incremental levels. Participants were asked to make fine-grained distinctions among these stimuli during fMRI scanning. Specifically, the paradigm required participants to appraise the level of threat conveyed by each stimulus, a process highly relevant to clinical anxiety (
5). This task was expected to engage the ventral prefrontal cortex, given the role of this region in threat-safety discrimination.
Previous research has suggested two hypotheses: adolescent anxiety disorders predict high risk for adult anxiety disorders, and exposure therapy appears to be similarly beneficial in the two age groups. We therefore hypothesized that anxiety disorders in the two age groups would manifest overlapping areas of prefrontal cortex dysfunction. However, research on the neural circuitry mediating normal types of fear has found age-related differences in prefrontal cortex function (
12). Moreover, only a subset of adolescent anxiety disorders persists into adulthood (
2). As a result, we also expected to observe age-related differences in prefrontal cortex function in both the healthy and anxious groups.
Method
Participants
Youths and adults participated in this study as paid volunteers. Written informed consent from adult participants and parents and written assent from youth participants were provided. Procedures were approved by the National Institute of Mental Health Institutional Review Board.
Individuals were studied if they were medication free, physically healthy, and had an IQ >70. All participants received a comprehensive psychiatric assessment: the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (
15) for adults and the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (
16) for youths under age 18. Parents and youths were interviewed separately, and discrepancies were reconciled with both. Healthy participants were free from any current axis I disorder. Participants with anxiety disorders met DSM-IV criteria for a primary diagnosis of either generalized anxiety disorder or social phobia. To be consistent with previous research, additional anxiety disorders and major depressive disorder were permitted; however, current or past diagnoses of posttraumatic stress disorder (PTSD) or obsessive-compulsive disorder or other comorbid disorders were exclusionary.
Procedures
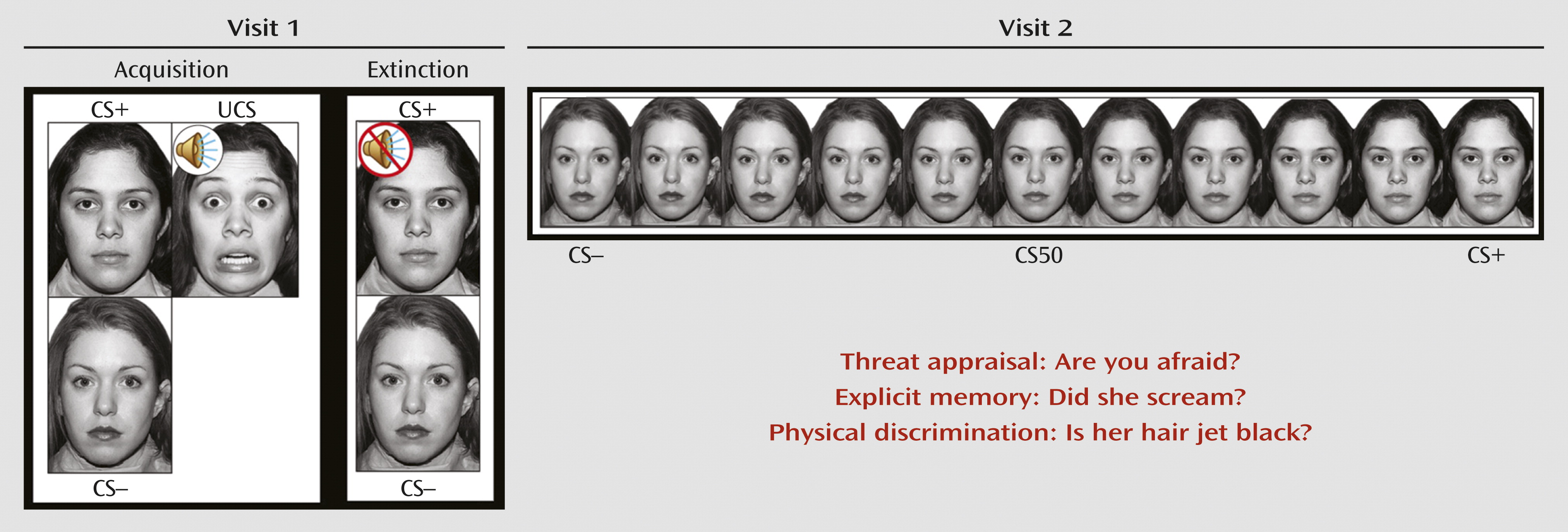
In this study, we adapted a differential fear conditioning and extinction paradigm used in earlier research (
13). During fear acquisition, individuals passively viewed neutral faces of two women, the CS. One woman, the CS+, predicted the UCS, a fearful face coterminating with an aversive scream, while the other woman, the CS–, did not. Participants were instructed that they could learn to predict when the UCS would occur but were not informed of the CS/UCS contingency. During extinction, the CS+ and CS– were presented repeatedly in the absence of the UCS (
Figure 1). Skin conductance response, startle response using electromyography, and self-reported anxiety were used to assess fear acquisition and extinction. Responses to the CS+, CS–, UCS, and interstimulus interval were measured (see the
data supplement that accompanies the online edition of this article).
Three weeks after fear conditioning and extinction (mean=19.7 days [SD=9.7]), participants underwent MRI scanning. The MRI sample included 14 anxious youths, 15 anxious adults, 25 healthy youths, and 28 healthy adults (
Table 1; see also the online
data supplement).
The fMRI paradigm was explicitly designed to capture precisely the transitional boundary between threat and safety through the use of morphed images (
17–
19) (
Figure 1). In blocks of trials, participants viewed the CS+ and CS– and nine morphed images consisting of different blends of the CS– and CS+. This stimulus array was used to measure response gradients (linear or quadratic trends) along a continuum (
19,
20). Previous research of recall in clinical anxiety has used passive viewing to mimic animal research; however, passive viewing does not engage psychological processes, which are particularly relevant to clinical anxiety, such as the internal focus on subjective fear levels (i.e., threat appraisal) (
5). Threat appraisal can be assessed and compared with other psychological processes by directing participants to focus on a particular question with regard to each stimulus presented. Therefore, for each block, participants used a button press to answer one of three yes or no questions in response to each face: 1) Are you afraid now? (threat appraisal); 2) Did she scream in the past? (explicit memory); and 3) Is her hair jet black? (perceptual discrimination).
Using linear mixed-effects, random-effects analysis in AFNI (Analysis of Functional NeuroImages [
http://afni.nimh.nih.gov/afni/]) (
21), statistical interactions were tested among between-group factors (diagnosis [anxious, healthy], age group [adult, youth]), within-group factors (cognitive instruction [threat appraisal, explicit memory, and physical discrimination]), and covariates (linear [morph level] and quadratic [morph
2-level] trends across morphed images). The linear and quadratic trend regressors consisted of the morphed level for each image and its square, respectively. Scanner and days between fear conditioning and scanning were included as covariates of no interest. Interactions were tested at the whole-brain level and in the ventral region of the medial prefrontal cortex, an a priori region. Because of their relevance to fear conditioning and extinction, the amygdala and the hippocampus were also examined. In our omnibus analysis, we corrected for multiple comparisons using a family-wise-error approach at the cluster level through Monte Carlo simulations. The cluster threshold probability was set at 0.05, with a voxel-wise p value of 0.005. Statistical significance was defined as 90 voxels (1,406 mm
3) for whole brain. To restrict the search territory, regional masks of ventral regions of the medial prefrontal cortex, encompassing both the subgenual anterior cingulate cortex and the ventromedial prefrontal cortex (11,453 mm
3, effectively a 14-mm radius sphere), and the amygdala and hippocampus (1,828 mm
3, effectively a 7-mm radius sphere) were used to determine the cluster threshold for a priori regions. Based on calculations, the cluster threshold was 15 voxels (234 mm
3) for the medial prefrontal cortex and three voxels (47 mm
3) for the amygdala and hippocampus. These thresholds were determined using the AFNI AlphaSim program (the methods are described in the online
data supplement).
Post hoc tests were conducted within each significant cluster identified by higher-level interactions (i.e., diagnosis-by-age group-by-instruction-by-morph
2 level). Percent-signal-change values, relative to baseline, were averaged across voxels within each significant cluster for each of the 33 effects of interest for all individual participants. Using these extracted values in SPSS (SPSS, Inc., Chicago), linear mixed-model analysis and post hoc tests with Bonferroni correction decomposed statistically significant group differences that emerged in the omnibus analysis. These post hoc tests determined group differences in activation levels and patterns of response (i.e., significant linear and quadratic trends) when participants were directed to different cognitive states (i.e., threat appraisal, explicit memory). Behavioral data were analyzed in a similar manner (see the
data supplement).
Results
Several noteworthy outcomes emerged following fear acquisition and extinction. These effects are summarized here, and further details are provided in the online
data supplement. First, more anxious than healthy individuals discontinued participation (diagnosis odds ratio: 4.2, p<0.02), as did more youths than adults (odds ratio for a 5-year increment in age: 10.3, p<0.001). Second, both anxiety disorder groups reported higher subjective ratings for both the CS+ and CS– than the two healthy groups (F=19.3, df=1, 109, p<0.001). During fear acquisition, both physiological and subjective measures revealed robust fear conditioning (CS+ > CS–), with similar levels in all groups (skin conductance response: difference between CS+ and CS–, mean=0.1, t=4.8, df=99, p<0.001; startle response: difference between CS+ and CS–, mean=2.0, t=4.3, df=113, p<0.001; subjective fear: difference between CS+ and CS–, mean=1.9, t=7.9, df=113, p<0.001; no significant group effects were found). Finally, during extinction, no diagnostic-group or age-group differences in conditioning levels were demonstrated in skin conductance response. However, anxiety-related differences were detected in subjective response (diagnosis-by-age group-by-phase-by-CS type interaction: F=4.5, df=2, 218, p<0.02) and age-related differences in startle response of conditioned fear (age group-by-phase-by-CS type interaction: F=2.6, df=4, 440, p<0.04).
Behavioral data were collected during scanning several weeks later. Across all groups, a quadratic pattern of online ratings across stimuli was noted for threat appraisal (β=0.003, SE=0.001, t=2.6, df=899, p<0.001) and explicit memory (β=0.008, SE=0.001, t=7.5, df=899, p<0.001), albeit weaker for threat appraisal (instruction-by-morph
2-level interaction: F=4.2, df=2, 1931, p<0.01). Although behavior differed based on cognitive instruction, there were no effects of diagnosis or age group on these ratings during scanning (see Figure S2 in the online
data supplement). Additional behavioral data are presented in the
data supplement.
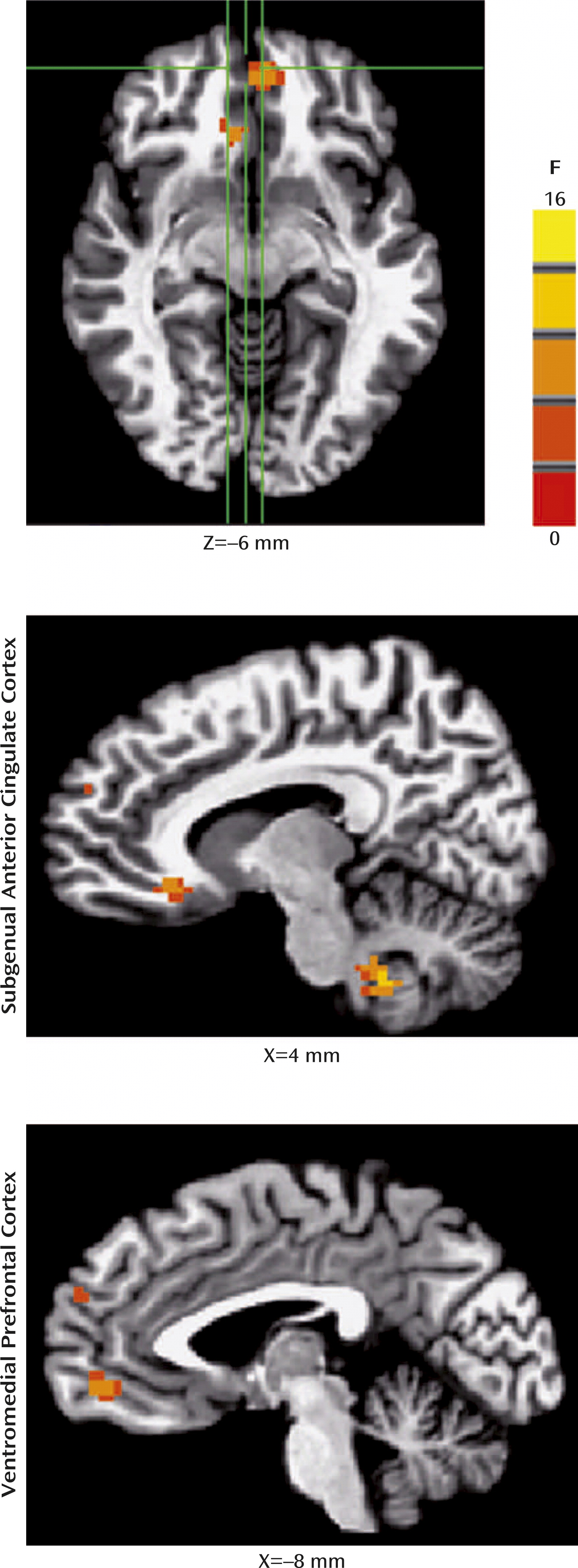
For the fMRI data, whole-brain random-effects analyses indicated four-way interactions (diagnosis-by-age group-by-instruction-by-morph
2 level) in two clusters within the medial prefrontal cortex: the subgenual anterior cingulate cortex/Brodmann’s area [BA] 25/BA 32 ([coordinates: –9, 26, –9], 29 voxels [453 mm
3], F=8.5, df=2, 2592, p<0.05, corrected); and the ventromedial prefrontal cortex/BA 32/BA 11 ([coordinates: 4, 49, –6], 43 voxels [672 mm
3], F=9.2, df=2, 2592, p<0.05, corrected) (
Figure 2). No other regions (i.e., the amygdala or hippocampus) survived a priori cluster-level or whole-brain cluster-level correction.
To examine higher-level interactions in the significant clusters, the average percent-signal change values, relative to baseline, from voxels within these clusters were extracted for individual participants. Data for each cognitive instruction were examined separately (i.e., threat appraisal, explicit memory) (see the online
data supplement).
Threat Appraisal
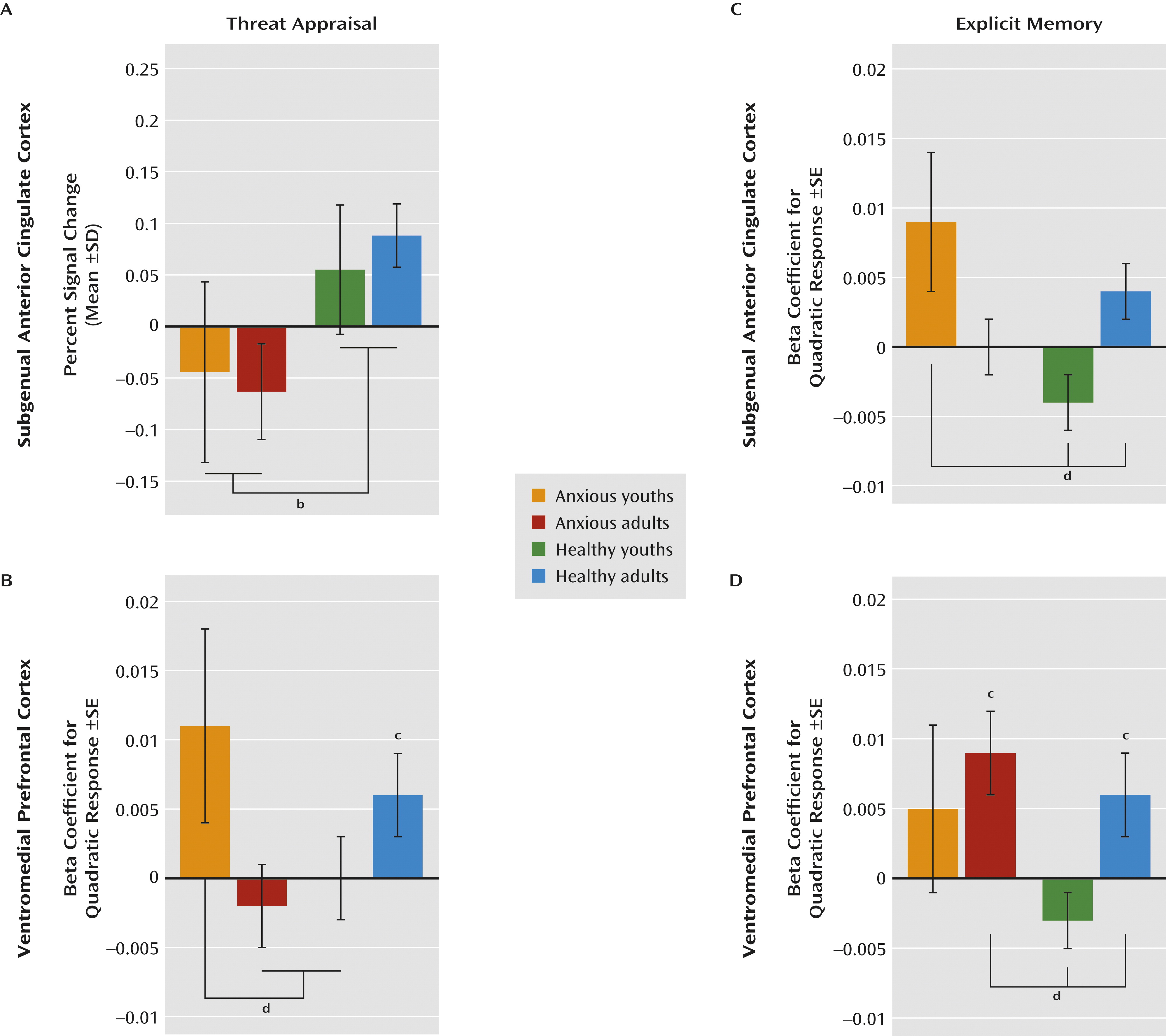
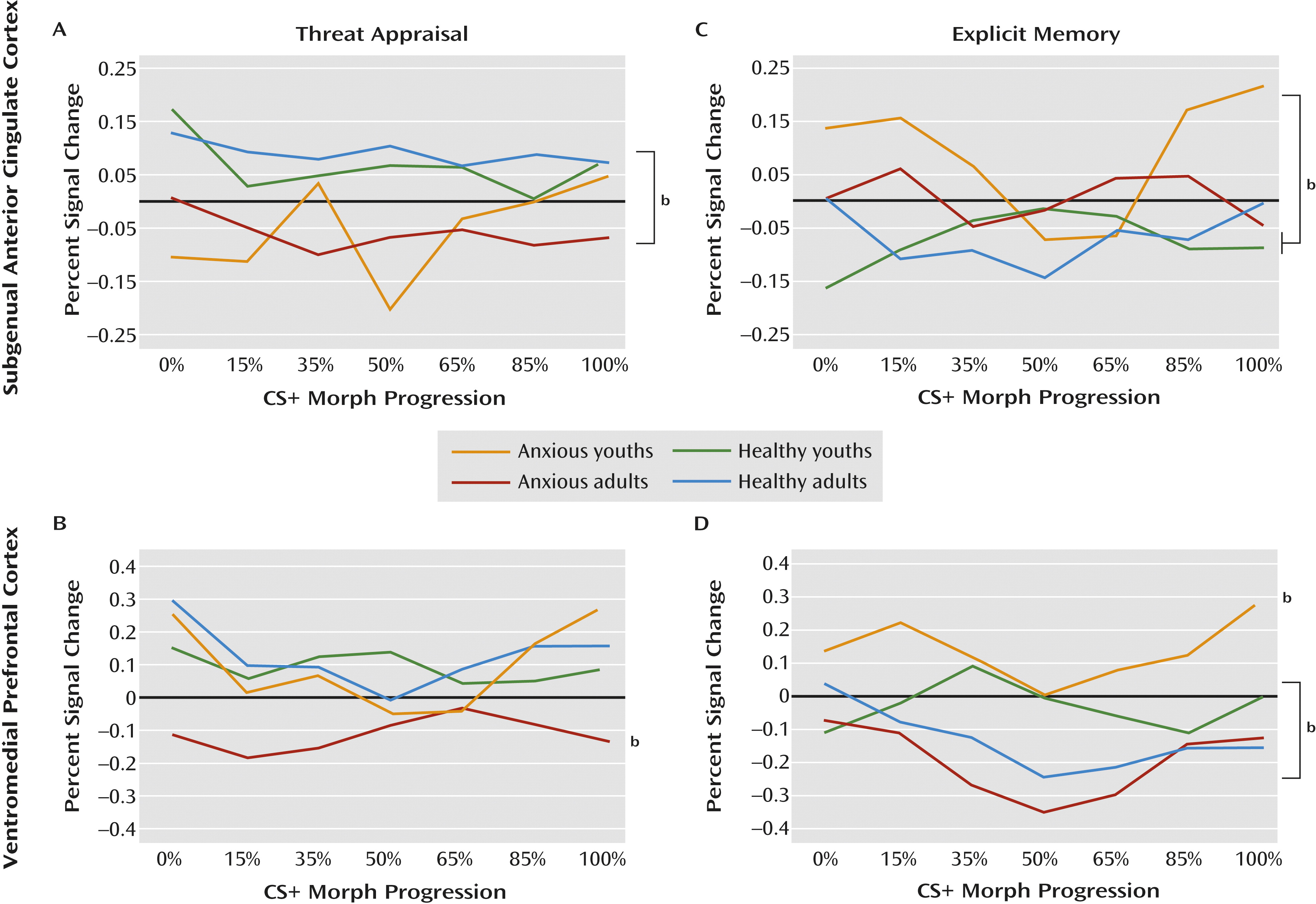
Patterns in the subgenual anterior cingulate cortex and the ventromedial prefrontal cortex differed when participants were instructed to indicate whether or not they were afraid (i.e., threat appraisal). Compared with healthy subjects, anxious patients exhibited subgenual anterior cingulate cortex hypoactivation during threat appraisal (full sample: F=12.1, df=1, 207, p<0.001; adults: F=9.1, df=1, 207, p<0.003; adolescents: F=3.7, df=1, 207, p<0.06), with no diagnosis group-by-age group interaction. The activation in the subgenual anterior cingulate cortex also did not vary across morphed images (
Figures 3A and
4A).
In contrast, activation in the ventromedial prefrontal cortex during threat appraisal varied with both clinical anxiety and development. First, anxious adults exhibited a unique pattern (diagnosis-by-age group: F=6.1, df=1, 479, p<0.02), revealing hypoactivation in the ventromedial prefrontal cortex compared with the other three groups (F=4.9, df=3, 188, p<0.003). Thus, for anxious adults, the pattern of activation in the ventromedial prefrontal cortex during threat appraisal resembled the pattern in the subgenual anterior cingulate cortex. Second, anxious youths exhibited a unique pattern across morphed images (diagnosis-by-age group-by-morph
2 level: F=4.3, df=1, 540, p<0.04). Unlike the subgenual anterior cingulate cortex, the pattern in the ventromedial prefrontal cortex in anxious youths differed from that observed in the ventromedial prefrontal cortex in anxious adults. Specifically, anxious youths manifested greater activation in response to the morphed images at the extremes of the continuum relative to the blended images compared with both healthy youths and anxious adults (both t values >2.2, df=2536, p<0.03) (
Figures 3B and
4B). Thus, anxious youths exhibited a stronger U-shaped response across the morphed images than these groups.
Explicit Memory
The main purpose of the analyses for explicit memory was to demonstrate that findings in threat appraisal, the more clinically relevant state, were task specific. Results suggested that the patterns described above were specific to the threat-appraisal task. When participants were instructed to remember the UCS (i.e., the explicit-memory task), patterns of activation in the subgenual anterior cingulate cortex differed from those observed during the threat-appraisal task. Specifically, anxious youths exhibited hyperactivation in the subgenual anterior cingulate cortex compared with both healthy groups (diagnosis-by-age group: F=5.2, df=1, 504, p<0.02; F=5.2, df=3, 226, p<0.002). Moreover, unlike during the threat-appraisal task, morph status affected activation in the subgenual anterior cingulate cortex during the memory task (diagnosis-by-age group-by-morph
2 level: F=6.4, df=1, 542, p<0.02). Specifically, quadratic effects of stimulus class differed between anxious and healthy youths (t=3.1, df=2667, p<0.002) and between healthy adults and healthy youths (t=2.2, df=2667, p<0.03) (
Figures 3C and
4C).
Task demands also affected the ventromedial prefrontal cortex, albeit with somewhat more evidence of similarities between the threat-appraisal and explicit-memory tasks than the subgenual anterior cingulate cortex. Thus, both age and diagnosis influenced ventromedial prefrontal cortex response on the explicit-memory task, as they did for the threat-appraisal task; nevertheless, the nature of the findings in the explicit-memory task differed from those in the threat-appraisal task. Specifically, anxious youths manifested signs of overall hyperactivation in the ventromedial prefrontal cortex compared with the other groups (F=10.2, df=3, 203, p<0.001). Additionally, healthy youths exhibited significantly less deactivation than anxious adults (p<0.05). Finally, unlike youths, both adult groups exhibited a quadratic pattern of response across the morphed images (both t values >2.4, df=162, p<0.02). This generated a significant difference in patterns across morphed images between healthy youths and both adult groups (both t values >2.3, df=2536, p<0.03) (
Figures 3D and
4D).
Discussion
In this study, clinically anxious and healthy adults and adolescents underwent fear conditioning and extinction in the clinic, followed by fMRI scanning while CS+ and similar-appearing stimuli were viewed in different attention states. In the clinic, the healthy and anxious groups differed on overall self-reported fear and on changes in self-reported fear between conditioning and extinction. In the MRI scan, two key findings emerged during events that required participants to make difficult threat-safety discriminations when appraising threat. First, compared with healthy individuals, anxious adults and adolescents both exhibited hypoactivation in the subgenual anterior cingulate cortex, suggesting the presence of a shared feature in adult and adolescent anxiety disorders. In contrast, there were age-specific findings in the ventromedial prefrontal cortex. Specifically, compared with age-matched healthy subjects, anxious adults exhibited less activation in the ventromedial prefrontal cortex, whereas anxious adolescents exhibited greater activation in this region in response to the most extreme CS+ and CS– (i.e., greater quadratic response).
Fear Conditioning and Extinction
Data on discontinuation rates and self-reported fear address the ecological validity of the paradigm. We observed high discontinuation rates among anxious youths, demonstrating the difficulty of studying fear in youths. While no adults discontinued, 49% of anxious youths and 14% of healthy youths did. Moreover, youths who discontinued tended to be more anxious than those who did not, suggesting that the paradigm evokes clinically relevant avoidance. Previous research using fear conditioning (
22,
23) and similarly aversive procedures in youths also found high discontinuation rates (
24,
25). Both anxious adults and youths reported more fear than their respective healthy counterparts in response to both the CS+ and CS–, across both conditioning and extinction. Moreover, compared with the healthy groups, the anxious groups reported less change in fear in response to the CS+ between conditioning and extinction. These findings also address the ecological validity. That is, had the anxious and healthy groups reported similarly low levels of fear, then the clinical relevance of the paradigm would be questioned. Alternatively, had one patient group reported consistently higher fear than the other, then these differences could have affected our fMRI findings.
During both conditioning and extinction, the pattern of results differed between the physiological data and self-reported fear. Such discrepant findings have appeared in previous studies (
12,
14). While the anxious and healthy groups differed on self-reported fear in our study, these groups exhibited no such differences on measures of physiology, either during conditioning or extinction. This fear-conditioning study is the first, to our knowledge, to include both anxious youths and anxious adults in a single study, but previous research of each age group alone found results similar to ours (
4,
13). That is, previous studies reported both between-group differences in overall levels of self-reported fear and the absence of differences in skin conductance response or startle response (
26,
27). Moreover, this discrepancy between self-reported fear and physiology is consistent with a wealth of other research showing that individual differences in reported fear correlate weakly, if at all, with individual differences in skin conductance response or startle response (
28,
29).
Shared Features
This study also demonstrated subgenual anterior cingulate cortex hypoactivation during threat appraisal in both adult and adolescent patients with anxiety disorders compared with their healthy counterparts. Such findings are consistent with data implicating the subgenual anterior cingulate cortex in emotion regulation and signs of deficient emotion regulation in PTSD. For instance, other studies of healthy adults have linked subgenual anterior cingulate cortex engagement to effective emotion regulation (
30,
31), and studies of PTSD have found lower subgenual anterior cingulate cortex function in patients than in healthy adults during exposure to an extinguished CS+ (
4).
Observations of subgenual anterior cingulate cortex hypoactivation in this study and in previous studies of anxious adults are notable, given cross-study differences in methodology. For example, shock was used as the UCS in previous studies of adults but not in our study because of concerns about potential harm to adolescents. Moreover, previous studies contrasted activation in the subgenual anterior cingulate cortex to extinguished versus nonextinguished CS+ (
4,
32). Rather than contrasting such distinct types of CS+, we used stimuli with various degrees of resemblance to a single CS+. Finally, whereas previous studies of adults used passive viewing, we employed an explicit task adapted from previous research, which was designed to demonstrate engagement of a disorder-relevant process. This decision followed best-practice recommendations for fMRI research in difficult-to-study populations, such as adolescents with anxiety disorders (
33). In any case, the observation of hypoactivation in the subgenual anterior cingulate cortex across methodologically different studies demonstrates the presence of a replicable association in adult anxiety disorders. Our study now extends this observation to adolescents.
By comparing adolescents and adults, this study bridges longitudinal findings on the outcome of adolescent anxiety disorders with fMRI findings on prefrontal cortical dysfunction in adult anxiety disorders. In longitudinal research, most adults with anxiety disorders also met criteria for an anxiety disorder in adolescence. Such findings suggest similar neural correlates in adolescent and adult anxiety disorders. As noted above, fMRI research in adults has found reduced subgenual anterior cingulate cortex function in the context of deficient fear learning, and our study is the first to directly compare fear learning in anxious and healthy adolescents and adults. It is important to do so, since healthy adolescents often differ from healthy adults in fMRI studies of emotional processes. For example, a recent study (
12) comparing healthy adults and adolescents using fMRI with the fear conditioning procedures we used here found that functioning differed in healthy adults and adolescents in both the amygdala and the prefrontal cortex.
Our research demonstrating subgenual anterior cingulate cortex dysfunction in both adolescents and adults with anxiety disorders is also relevant to research using both animal models and novel therapeutics. For example, rodent studies have found that lesions to the infra-limbic cortex, a possible rodent homologue of the subgenual anterior cingulate cortex, interfere with extinction (
7,
34). Such findings have generated interest in novel treatments for adult anxiety disorders that may reduce anxiety through increasing subgenual anterior cingulate cortex function; our findings suggest that such treatments may also be effective in adolescent anxiety disorders.
Age-Specific Features
Another result of this study concerns age-specific dysfunction in the ventromedial prefrontal cortex. Whereas anxious adults exhibited hypoactivation in the ventromedial prefrontal cortex during threat appraisal across the range of stimuli, anxious adolescents exhibited enhanced ventromedial prefrontal cortex response to the most extreme CS+ and CS–. This pattern could reflect heightened sensitivity in anxious adolescents in response to novelty, predictability, safety (
26), or dissimilarity (
19), especially when appraising threat.
These data raise questions about other differences between adolescent and adult anxiety disorders. As noted above, adolescent anxiety disorders predict high risk for adult anxiety disorders. Nevertheless, there is also discontinuity in longitudinal outcome. While the overwhelming majority of adults with an anxiety disorder suffered from an anxiety disorder in adolescence, only about one-half of adolescents with an anxiety disorder continue to manifest anxiety as adults (
2). This divergence suggests that adolescent anxiety disorders are heterogeneous and include subgroups of individuals who will mature to account for the majority of adult anxiety disorders and other subgroups who will remit. Differences in ventromedial prefrontal cortex function among anxious adolescents and adults could relate to these varying longitudinal trajectories. For example, changes in ventromedial prefrontal cortex function from adolescence through adulthood could influence anxiety symptom trajectories, thus mediating the adult outcome of adolescent anxiety disorders. Alternatively, the level of ventromedial prefrontal cortex dysfunction present in adolescence could define distinct subgroups of anxiety disorders and predict outcome. For example, the subgroup of anxious adolescents with ventromedial prefrontal cortex hypoactivation, a deficit characteristic of adult disorders, may be at particularly high risk for persistence. Other adolescents who exhibit elevated activation may experience a better long-term outcome. As noted in
Figure 3B, activation in the ventromedial prefrontal cortex in anxious youths demonstrated a quadratic pattern during the threat-appraisal task, albeit with marked variability. The subgroup of anxious youths with particularly low activation (i.e., those whose activation overlaps with that of anxious adults) may face high risk for adulthood anxiety.
Limitations
Several limitations of our study should be mentioned. First, the broad age range of the participants may obscure developmentally sensitive processes (
35). For example, evidence suggests that it may be more difficult to extinguish conditioned fear in early adolescence compared with late adolescence (
34). Second, the youngest participants were the least likely to complete the procedures, raising questions about the degree to which comparable findings would occur with more tolerable procedures for younger patients. However, to generate comparable findings, it would be important for future research with alternative procedures to elicit robust group differences in avoidance and subjective fear, as did the paradigm in this study. Third, while group differences emerged in prefrontal cortical function, corresponding differences in task-elicited behavior were not found. Some investigators view the absence of such differences as a limitation, whereas others argue that important experimental confounders are removed (
33). Fourth, data on peripheral physiology were acquired only during the first visit. Future studies should acquire physiological and neural data simultaneously, although including physiological data collection would lengthen the fMRI procedures and potentially reduce their tolerability. Finally, while the anxious and healthy groups were matched on key variables, IQ differed between adolescents and adults. Nevertheless, IQ showed no associations with any dependent measure, and group differences in brain function persisted in subgroups matched on both age and IQ.
Conclusions
No previous fMRI study has compared healthy and anxious adolescents and adults in the context of a fear-learning paradigm. This study specifically examined threat appraisal and explicit memory during exposure to CS+ in adolescents and adults following conditioning and extinction. Using a paradigm that evokes elevated fear in adolescents and adults with an anxiety disorder, we found hypoactivation in the subgenual anterior cingulate cortex in anxious adolescents and adults compared with healthy adolescents and adults. Such hypoactivation occurred in both age groups specifically when appraising the fear response. In contrast, activation in the ventromedial prefrontal cortex differentiated anxious adults and adolescents both from each other and their age-matched healthy counterparts.
Acknowledgments
The authors thank Marilla Geraci for assistance with recruitment and David Luckenbaugh for assistance with statistical data.