Irritability can be defined as a low threshold for experiencing negative affect in response to frustration, with frustration being the emotional response to blocked goal attainment (
1,
2). Irritability is both common and impairing in child and adult psychiatric disorders (
3), and its importance is recognized in DSM-5 with the adoption of disruptive mood dysregulation disorder, a disorder whose defining feature is excessive and impairing irritability. Given the importance and pervasiveness of irritability, it is notable that few neuroimaging studies have been conducted to examine its pathophysiology. Since irritability can be viewed as a decreased threshold for experiencing frustration, one approach to studying its pathophysiology is by evoking frustration in the scanner. In this study, we compared the neural correlates of frustration in healthy children and children with chronic and impairing irritability.
Frustration is typically induced in the laboratory by using paradigms that increase task difficulty or deceive participants into believing that because their performance is substandard, they cannot earn a reward (
4–
10). The few functional MRI (fMRI) studies that have investigated the circuitry mediating frustration have implicated the amygdala, parietal attentional networks, and the dorsal and ventromedial prefrontal cortex. Healthy adults exhibit increased amygdala activation with increased frustration (
11), consistent with the amygdala’s role in detecting emotional salience (
12). Healthy children demonstrate an increase in a parietally mediated attentional event-related brain potential (
9,
10) and greater dorsal and ventromedial prefrontal cortex recruitment during frustration (
4), perhaps reflecting the engagement of attentional or cognitive resources to facilitate task performance or emotion regulation. Finally, frustration tasks may activate the ventral striatum, given its ability to signal when an expected reward is not received (negative prediction error) (
13,
14).
The present study is, to our knowledge, the first fMRI study to examine neural responses to frustration in healthy children and children with severe, chronic, and impairing levels of irritability (operationalized as severe mood dysregulation [
1,
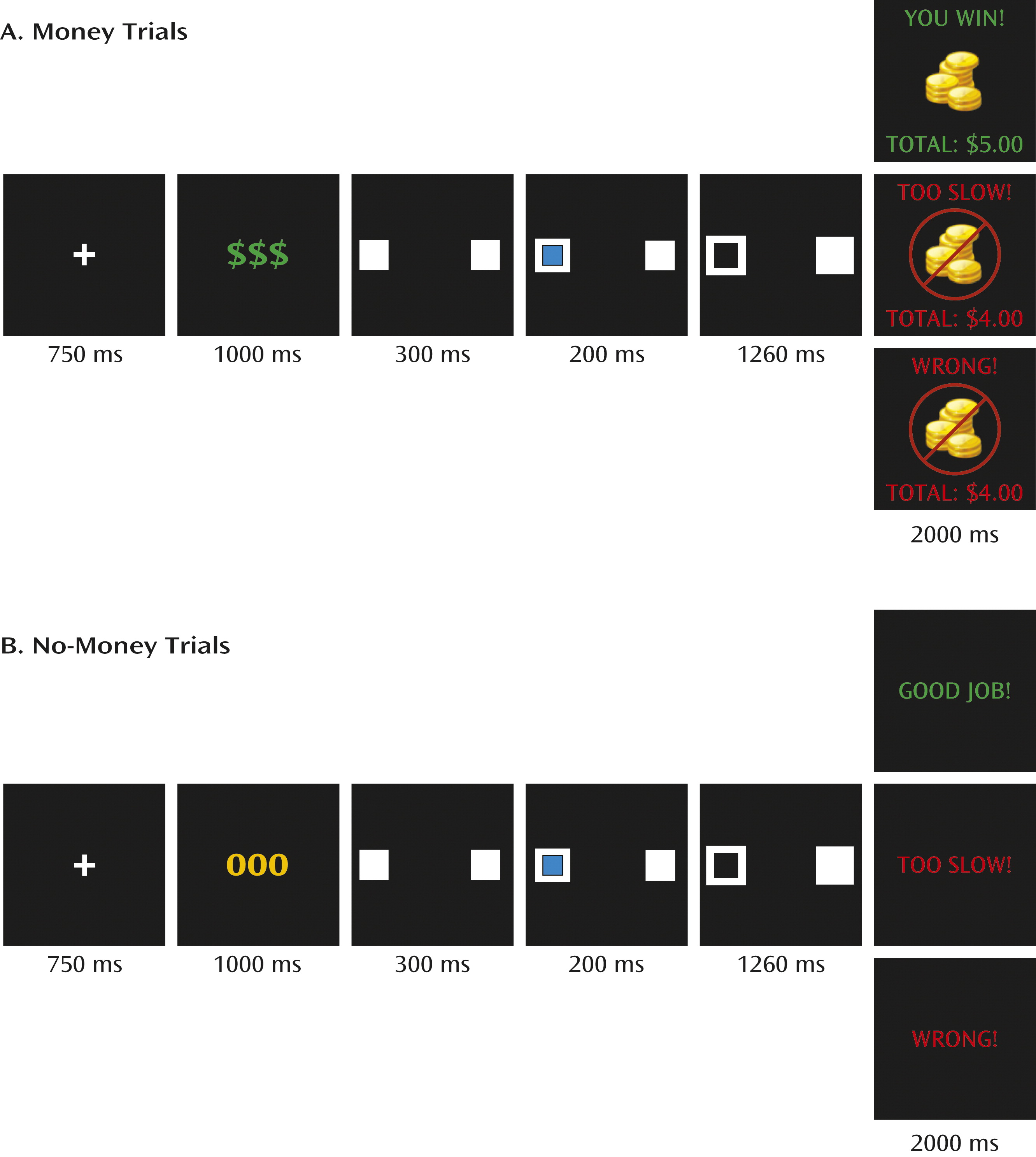
2]). Although this study predated the definition of disruptive mood dysregulation disorder, all of the children with severe mood dysregulation in this study would meet criteria for this disorder. Children with severe mood dysregulation and healthy children completed an adaptation of the Posner spatial cuing task that included monetary rewards (
7–
10). Frustration was induced by telling participants that they were responding too slowly and therefore losing money. We hypothesized that during frustration, children with severe mood dysregulation would exhibit, relative to healthy children, increased amygdala activation, decreased activation in prefrontal regions responsible for cognitive and emotion regulation, decreased parietal activation, and abnormal activation in the ventral striatum reflecting aberrant prediction errors.
Results
Participants
A total of 61 children enrolled, 19 of whom were excluded for various reasons (inability to complete the task because of frustration or anxiety, five children with severe mood dysregulation; not deceived, two healthy children; technical difficulties, one child with severe mood dysregulation and one healthy child; structural brain abnormality, one healthy child; insufficient numbers of trials/condition (<35% of trials) because of poor behavioral performance or motion >2 mm, five children with severe mood dysregulation and three healthy children; and poor coregistration, one healthy child). The final sample included 19 children with severe mood dysregulation (seven of them unmedicated) and 23 healthy comparison children (
Table 1). The groups did not differ significantly in age or full-scale IQ. The severe mood dysregulation group had a greater proportion of boys than the healthy comparison group (χ
2=4.27, p<0.04), so sex was a covariate in all analyses.
Affect Ratings
A group-by-time interaction (F=3.42, df=5, 190, p<0.05) revealed that children with severe mood dysregulation reported more frustration than healthy children after the last two runs of game 3, but not at earlier assessment points. All participants felt more unhappy during the frustration condition (game 3) than during nonfrustration conditions (games 1 and 2) (time, F=12.76, df=5, 190, p<0.001), but there were no main effects of group or interactions. No findings emerged from the arousal ratings.
Behavioral Results
The first behavioral analysis (group-by-time-by-validity) revealed a time-by-validity interaction for response time (F=7.67, df=5, 195, p<0.001) and accuracy (F=30.05, df=5, 195, p<0.001). In general, participants were faster but less accurate during frustration relative to nonfrustration trials, with no differences between groups.
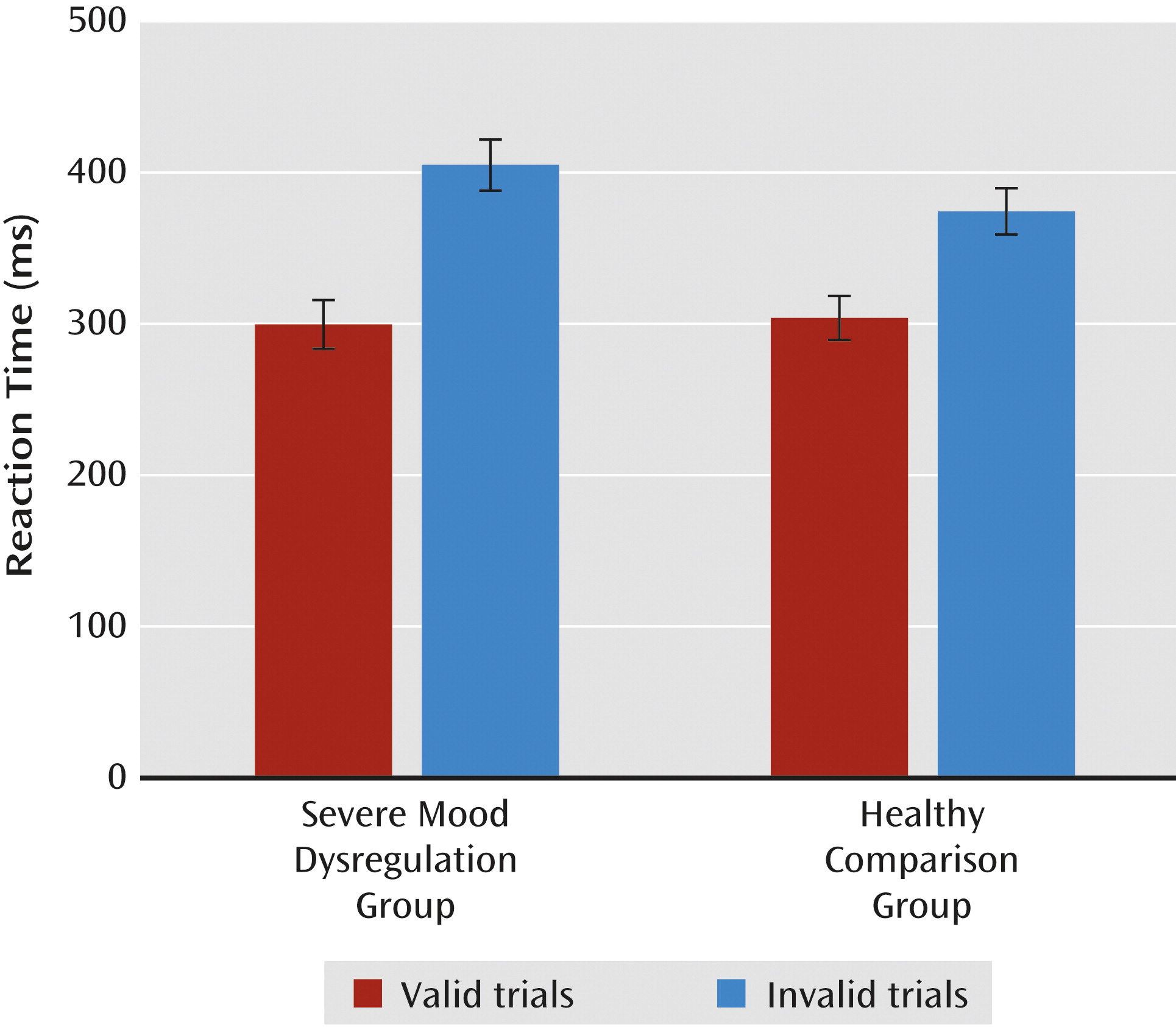
The second behavioral analysis (group-by-validity-by-trial type), was restricted to the frustration condition (game 3), since only game 3 included money and no-money trials. On response time, there was a group-by-validity interaction (F=5.41, df=1, 39, p<0.03). During frustration, all participants responded more slowly during invalid than valid trials; however, children with severe mood dysregulation were slower than healthy children only on invalid trials (
Figure 2). A main effect of trial type also emerged (F=8.24, df=1, 39, p<0.01), indicating that participants responded more quickly during money than no-money trials. No group differences emerged for accuracy.
fMRI Activation Results
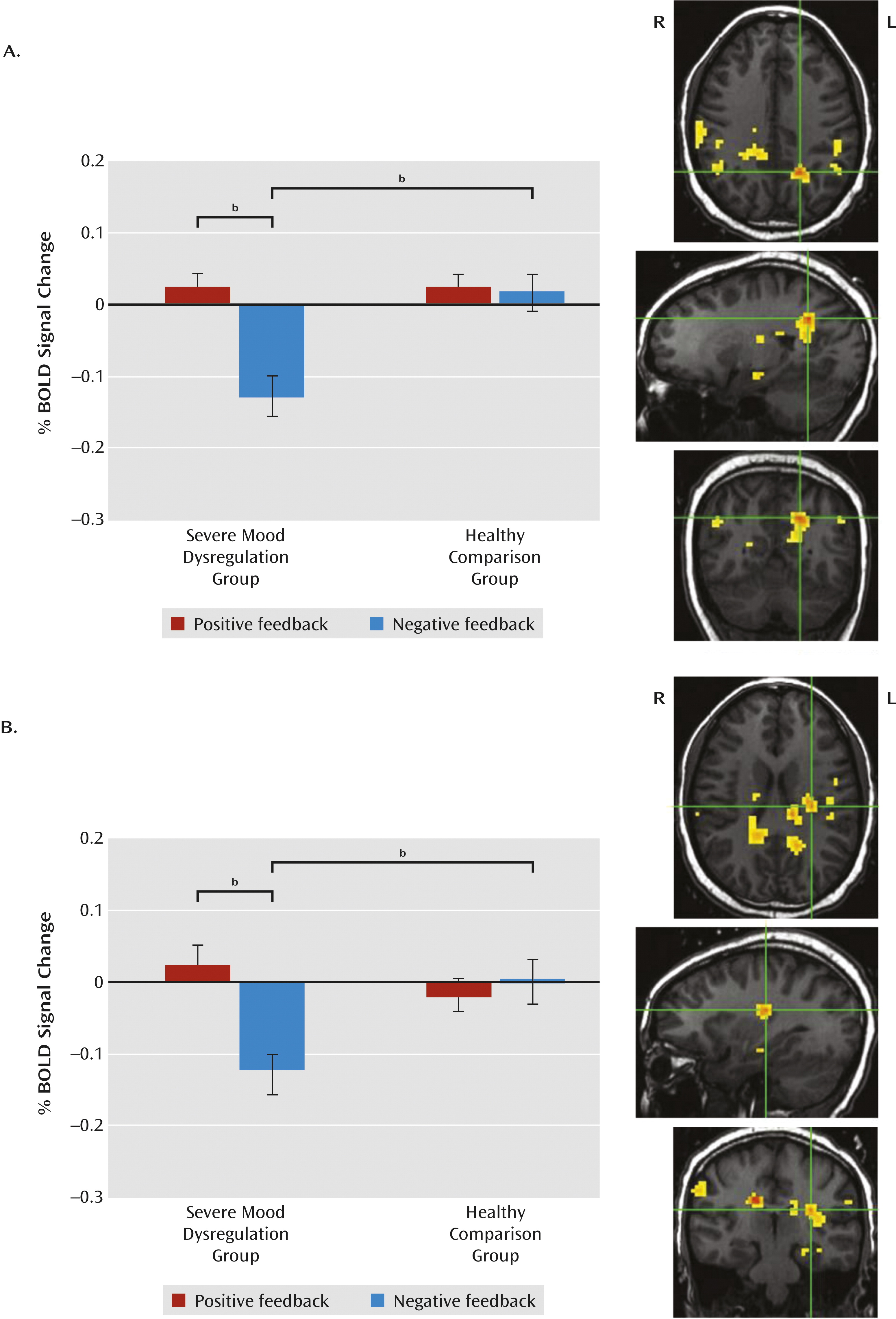
The amygdala region-of-interest analysis revealed a group-by-feedback-by-hemisphere interaction (F=4.70, df=1, 39, p<0.04). Compared with healthy children, children with severe mood dysregulation exhibited less activation in the left amygdala on negative feedback trials (between-group difference). There was also a within-group difference in the severe mood dysregulation group only, with participants exhibiting less activation in the left amygdala on negative relative to positive feedback trials. Activation in healthy children did not differ between positive and negative feedback trials, and activation did not differ between groups on positive feedback trials.
In the striatum, the group-by-trial type-by-feedback-by-hemisphere-by-region ANCOVA revealed a group-by-feedback interaction (F=5.54, df=1, 39, p<0.03) but no higher-order interactions. The pattern in the striatum was similar to that in the amygdala: children with severe mood dysregulation exhibited less activation in the left and right striatum than healthy children during negative feedback trials (between-group difference). Children with severe mood dysregulation also exhibited less activation in the striatum during negative relative to positive feedback trials. This within-group difference was present in the severe mood dysregulation group only; in healthy children, striatal activation did not differ between positive and negative feedback trials. As in the amygdala, children with severe mood dysregulation did not differ from healthy children on striatal activation during positive feedback trials.
In the whole-brain analysis, no regions showed a group-by-trial type-by-feedback interaction. However, there was a group-by-feedback interaction in 11 regions (
Table 2;
Figure 3). Activation patterns were identical in each region and similar to the region-of-interest results—that is, we observed a between-group difference during negative feedback trials, no group differences during positive feedback trials, and, in the severe mood dysregulation group only, a within-group difference on negative relative to positive-feedback trials. Specifically, relative to healthy children, children with severe mood dysregulation exhibited less activation in response to negative feedback in parietal, parahippocampal, and thalamic/cingulate/striatal regions (F values, >10.00, p values, <0.005). Activation did not differ between groups on positive feedback trials. In these same regions, children with severe mood dysregulation exhibited less activation in response to negative relative to positive feedback (F values, >7.50, p values, <0.02), whereas healthy children did not show a within-group difference.
Exploratory Analyses
Post hoc analyses (see Table S3 in the online
data supplement) indicated that unmedicated children with severe irritability, as well as children with severe mood dysregulation without comorbid ADHD, exhibited hypoactivation relative to healthy children in the left amygdala, in the left and right striatum, and in 10 of the 11 regions identified by the whole-brain analyses (F values, >4.10, p values, <0.05).
In both children with severe mood dysregulation and healthy children, greater self-reported frustration during the task was associated with reduced activation in response to negative feedback trials in several regions identified in primary analyses (severe mood dysregulation: left inferior parietal lobule/supramarginal gyrus; right supramarginal gyrus; and left parahippocampal gyrus [r values, <−0.47, p values, <0.05]; healthy comparison children: left insula, left parahippocampal gyrus, left pre-/postcentral gyrus, right precuneus, and left cingulate/thalamus/caudate; left amygdala [r values, >−0.42, p values, <0.05]).
In healthy children, greater parent-reported irritability was associated with decreased accuracy on valid trials during frustration (r=−0.46, p<0.05), and greater self-reported irritability was negatively associated with activation to negative feedback trials in the left parahippocampal gyrus (r=−0.78, p<0.001). Self-reported irritability was not associated with any behavioral or neural measure in children with severe mood dysregulation.
Discussion
To our knowledge, this is the first fMRI study to compare the affective, behavioral, and neural correlates of frustration in children with severe and chronic irritability and healthy subjects. During the frustrating game, chronically irritable children exhibited behavioral deficits relative to healthy comparison children, in that children with severe mood dysregulation responded more slowly than healthy comparison children on invalid trials. Also during the frustrating game, irritable children differed from healthy children by showing marked deactivation of neural regions associated with spatial attention, reward processing, and emotional salience on the contrast of negative versus positive feedback trials. Groups did not differ in their neural response to positive feedback trials.
Both groups showed approximately 30% lower accuracy on invalid trials in the frustration versus nonfrustration condition.
Therefore, frustration decreased spatial attention flexibility in both groups, perhaps by inhibiting disengagement from the cue. Whereas the groups did not differ on accuracy during frustration, there were between-group differences on reaction time. Specifically, children with severe mood dysregulation were slower than healthy comparison children on invalid trials during frustration, suggesting they had particular difficulty shifting spatial attention away from the cue. Such behavioral deficits may be associated with the parietal hypoactivation observed in children with severe mood dysregulation in response to negative feedback, given the prominent role of parietal regions in mediating spatial attention processes (
19). Attention allocation skills are important for successful emotion regulation (
20), and deficits in attention control have been associated with increased negative affect and aggression in children (
21–
24). When frustrated and confronted with a negative event, children with severe mood dysregulation may have difficulty disengaging attention from the blocked goal and attending instead to stimuli that might serve as helpful distractors and improve their emotional state (
20). Alternatively, an inability to shift attention flexibly may reduce the number of emotion regulation strategies that can be identified and employed. Either mechanism might contribute to the irritable outbursts characteristic of children with severe mood dysregulation.
Alternatively, the behavioral impairments and frustration experienced by children with severe mood dysregulation may stem from deficits in bottom-up processes mediated by the amygdala/insula or striatum. Our observation of left amygdala hypoactivation in children with severe mood dysregulation during negative feedback is surprising, given the amygdala’s role in responding to emotionally salient stimuli (
12) and prior evidence of amygdala hyperactivation to frustrating stimuli in adults with high trait anger (
25,
26). However, an independent sample of children with severe mood dysregulation exhibited amygdala hypoactivation in response to emotional stimuli on another task (
15), perhaps reflecting generalized amygdala dysregulation, with a tendency toward hypoactivation, in chronically irritable children. Additional studies are needed to replicate and clarify these findings.
Decreased striatal response in children with severe mood dysregulation during negative feedback trials may reflect abnormalities in regions supporting reward processing. For example, negative prediction errors, which occur when an outcome is worse than expected, are associated with ventral striatal deactivation (
13,
14). Therefore, our striatal finding in children with severe mood dysregulation may indicate that these children experienced the frustrating event as more unexpected and aversive than did healthy children. This response may contribute to their exaggerated and inappropriate responses to frustrating events.
In contrast to two adult studies using similar frustration tasks (
25,
26), we did not observe group differences in prefrontal regions. Null findings are difficult to interpret, as they may reflect type II error. Indeed, children with severe mood dysregulation have been found to exhibit abnormal anterior cingulate activation (measured by magnetoencephalography) in response to frustrating feedback (
7) and inferior frontal gyrus activation deficits during an fMRI reward study (
16). Thus, deficits in prefrontal regions supporting top-down processing in response to frustration may exist in this population even though they were not observed in this study.
In interpreting the deactivation that we observed, frustration tasks probably induce longer-lasting emotional responses than those elicited by the presentation of, for example, emotional faces or pictures. Thus, it is possible that feelings of frustration remained “on” throughout all of game 3, in which case fixation trials may not have been cognitively or affectively neutral. This may complicate the interpretation of neural responses to task-related activity, since these are calculated relative to fixation trials. However, neural response to both positive and negative feedback trials were calculated with respect to fixation trials, and deactivation occurred only during negative trials. We are currently comparing neural responses to feedback under nonfrustration and frustration conditions to examine whether participants’ “baseline” activation changes during frustration.
Our findings in children with severe mood dysregulation are likely to be informative about neural dysfunction in children with disruptive mood dysregulation disorder during frustration. Like those with severe mood dysregulation, children meeting criteria for disruptive mood dysregulation disorder experience recurrent, excessive, and developmentally inappropriate temper outbursts, as well as chronic negative affect between outbursts. Unlike in severe mood dysregulation, the diagnostic criteria for disruptive mood dysregulation disorder do not include hyperarousal symptoms. Therefore, severe mood dysregulation can be viewed as a subset of disruptive mood dysregulation disorder. Since we do not know the degree to which irritability, rather than hyperarousal, is responsible for the abnormalities observed in children with severe mood dysregulation, it is unclear whether our findings would generalize to those with disruptive mood dysregulation disorder who do not have hyperarousal or ADHD symptoms.
Indeed, given high rates of co-occurring ADHD in children with severe mood dysregulation, behavioral and neural deficits related to attention are not surprising and may be related to ADHD symptoms rather than irritability. Post hoc analyses of the deactivation patterns observed in the irritable children in this study suggest that this is not the case. However, a stricter test would involve comparing children with severe mood dysregulation to nonirritable children with ADHD. We are currently conducting such a study. Similar research is necessary to evaluate the contribution of other clinical factors related to severe mood dysregulation (e.g., other co-occurring disorders, psychotropic medications) to confirm the specific effects of irritability. In addition, whether the chronic irritability of children with severe mood dysregulation affects neural and behavioral responses to frustration differently than the episodic irritability characteristic of children with bipolar disorder is unknown and deserves further study.
Our study has some limitation. First, previous studies using the affective Posner paradigm employed methods that isolated neural responses to feedback. In this study, the paradigm modeled the entire trial and thus limited our ability to detect neural activation specific to feedback. Second, behavioral findings, collected across all three games, suggest that attention flexibility differed between nonfrustration and frustration conditions. However, because of time constraints, fMRI data were collected only during the frustration condition (i.e., game 3). An ongoing study includes scanning during frustration and nonfrustration conditions. Third, high rates of psychotropic medication use and of comorbid diagnoses in children with severe mood dysregulation complicate attributions of study findings to irritability specifically. Exploratory post hoc analyses suggest that deficits in irritable children were not attributable to co-occurring ADHD or to psychotropic medications, but additional research is needed to identify the role of irritability independent of other clinical factors. Similarly, an important question for further research is the degree to which state frustration and trait irritability influence behavioral and neural responses to frustration. Finally, the task we used assessed only participants’ ability to shift spatial attention. Frustration likely influences other types of attention and perhaps cognitive control more broadly, and these are important issues to pursue in future research.