Social anxiety disorder is one of the most common forms of anxiety disorder (
1).
Clinical trials have demonstrated only moderate efficacy for anxiolytic pharmacotherapy (e.g., paroxetine, fluoxetine, and phenelzine) and cognitive-behavioral therapy (CBT) (2–5). Attempts to boost treatment response by combining CBT and anxiolytic pharmacotherapy for social anxiety disorder have met with disappointing results (6, 7), with one exception (
8).
Recently, a novel strategy has emerged for the combination of pharmacotherapy and CBT to improve outcomes. This strategy is the result of research studies that have mapped some of the core pathways involved in fear extinction (
9).
Fear learning and extinction are both blocked by antagonists at the glutamatergic N-methyl-d-aspartate (NMDA) receptor. d-Cycloserine, an analogue of d-alanine and a partial agonist at the NMDA receptor, has been found to augment learning in animals and in some human trials (
10). Moreover, the process of extinction of conditioned fear is facilitated by
d-cycloserine administered in individual doses prior to or soon after extinction trials in animals (
11–
13). Use of acute dosing as opposed to chronic dosing of
d-cycloserine may be critical to its intended effect on NMDA receptor activity (
14–
16).
The efficacy of
d-cycloserine in animal models led to the application of
d-cycloserine for humans, because exposure-based treatments in humans, such as CBT, rely on extinction to treat the core fears underlying anxiety disorders (
17,
18). Some preliminary studies have reported that
d-cycloserine augmentation effects of CBT, as compared with placebo, may be effective for a variety of anxiety disorders (
19), including social anxiety disorder (
20,
21). Other studies have reported weaker or no effects of
d-cycloserine as a CBT augmentation strategy for treating posttraumatic stress disorder (PTSD) (
22,
23) and obsessive-compulsive disorder (OCD) (
16,
24,
25). Two recent studies with patients with OCD (
24,
26) and a trial with PTSD patients (
22) suggest that
d-cycloserine may speed response to treatment rather than fundamentally change the outcome of treatment (
14,
27,
28).
The interpretation of this literature is complicated by small sample sizes and by inconsistencies in study design, target disorder, and administration schedules of
d-cycloserine. The most promising results thus far have come from preliminary studies that administered 50 mg of
d-cycloserine 1 hour before an abbreviated form of CBT (four to five sessions) delivered to small samples of patients with social anxiety disorder (
20,
21). Therefore, we adopted the same drug dosing and dosing schedule that were used in these earlier trials. However, the results of these proof-of-concept studies are of limited practical relevance, and many patients remain symptomatic after such brief treatments. To determine whether
d-cycloserine improves the speed or quality of response to a full course of CBT for social anxiety disorder, we designed this study to examine the short-term and long-term effects of 50 mg of
d-cycloserine administered acutely during the course of comprehensive CBT for social anxiety disorder in an adequately powered, larger-scale, double-blind, randomized, placebo-controlled trial. We hypothesized that
d-cycloserine would facilitate CBT for social anxiety disorder, resulting in faster treatment gains and greater treatment response and remission rates at the posttreatment and follow-up assessments as compared with placebo-augmented CBT.
Method
Participants
Participants were recruited between September 2007 and June 2011 through referrals to the three study sites (Boston University [BU], Massachusetts General Hospital [MGH], and Southern Methodist University [SMU]), from other area clinical facilities and programs, and through advertisements. Participants had to be at least 18 years of age; have a current DSM-IV diagnosis of generalized social anxiety disorder that the patient designated as the most important source of current distress or interference; have a score ≥60 on the Liebowitz Social Anxiety Scale (
29); have no clinically significant abnormalities based on physical examination, ECG, and laboratory findings; and be willing and able to comply with the requirements of the study protocol. Exclusion criteria included a lifetime history of bipolar disorder, schizophrenia, psychosis, delusional disorders, or OCD; an eating disorder in the past 6 months; organic brain syndrome, mental retardation, or other cognitive dysfunction that could interfere with the capacity to engage in therapy; a history of substance abuse or dependence (other than nicotine or caffeine) in the past 6 months or otherwise unable to commit to refraining from alcohol use during the treatment period of the study; PTSD within the past 6 months (entry of patients with other mood or anxiety disorders was permitted if the social anxiety disorder was judged to be the predominant disorder, in order to increase accrual of a clinically relevant sample); significant suicidal ideation as suggested by a score ≥3 on item 10 of the Montgomery-Åsberg Depression Rating Scale (
30) (or presence of suicidal behaviors within the past 6 months; concurrent psychotropic medication (e.g., antidepressants, anxiolytics, beta-blockers) for at least 2 weeks prior to entering the study; significant personality dysfunction likely to interfere with study participation; serious medical illness or instability for which hospitalization may be likely during the following year; any history of seizures; pregnant or lactating women and women of childbearing potential who are not using medically accepted forms of contraception; any concurrent psychotherapy initiated within 3 months of baseline or ongoing psychotherapy of any duration directed specifically toward treatment of social anxiety disorder (prohibited psychotherapy included CBT or psychodynamic therapy focusing on exploring specific, dynamic causes of the phobic symptomatology and providing management skills; general supportive therapy initiated more than 3 months before baseline was acceptable); prior nonresponse to adequately delivered exposure (as defined by the patient’s report of receiving specific and regular exposure assignments as part of a previous treatment); a history of head trauma causing loss of consciousness, seizure, or ongoing cognitive impairment; current treatment with isoniazid; and inability to understand study procedures and participate in the informed consent process.
Study participants underwent a two-step screening evaluation, consisting of an initial telephone screen and then a psychiatric assessment, medical history, and physical examination. (A CONSORT diagram of participant flow is available in the
data supplement that accompanies the online edition of this article). Patients in the
d-cycloserine-augmented CBT group did not differ from the placebo-augmented CBT group in mean age (34.6 years and 30.5 years, respectively).
Table 1 summarizes other demographic information for patients who were assigned to a treatment group. The two treatment groups did not differ in any of the demographic data at baseline, except that there were more males in the
d-cycloserine group than in the placebo group (64% and 49%, respectively; two-tailed Fisher’s exact test, p=0.045).
Study Design
Eligible participants were enrolled in a 12-session CBT protocol across three study sites (BU, MGH, and SMU), all using identical study protocols. One of the sites (MGH) is well known for conducting pharmacological studies, whereas the SMU and BU sites specialize in providing psychological interventions. At session 3, patients were randomly assigned to receive oral administration of either 50 mg of
d-cycloserine (N=87) or pill placebo (N=82) 1 hour prior to the start of sessions 3–7. Assignment to treatment condition was determined by a computer-generated allocation schedule with stratification by baseline severity of social anxiety disorder (i.e., Liebowitz Social Anxiety Scale score <70 or ≥70) (
29). Assessments of primary outcome variables were conducted at baseline, at sessions 2 through 8, at sessions 10 and 12, after treatment (week 13), and at 1-month, 3-month, and 6-month follow-ups (weeks 17, 25, and 37, respectively). The trial was sufficiently powered to detect a medium-sized effect (d=0.5) at 85% power and a significance threshold of 0.05 at the posttreatment (week 13) assessment. It was also sufficiently powered to detect a medium-sized group difference (d=0.5) at 80% power and a significance threshold of 0.05 at the 6-month follow-up, which was the assessment with the smallest number of participants available for the analyses. The protocol was approved by the institutional review boards of the three participating sites. Participants provided written informed consent after receiving a complete description of the study.
Assessment Measures and Procedures
Initial diagnoses were performed by trained study therapists using the Structured Clinical Interview for DSM-IV (
31) at the MGH and SMU sites and the Anxiety Disorders Interview Schedule for DSM-IV (
32) at the BU site. In addition, the Liebowitz Social Anxiety Scale was administered to assess for the generalized social anxiety disorder diagnostic subtype specifier. The blinded assessments were conducted by a master’s- or doctoral-level clinician trained in these assessments. The integrity and reliability of the diagnostic and efficacy evaluations was established through training by the MGH site and maintained by providing the clinicians with weekly supervision and feedback based on approximately 20% of the audiorecorded assessment interviews.
The primary outcome measures were Clinical Global Impression ratings tailored to social anxiety as assessed by the Liebowitz Social Anxiety Scale rating scale (
29) and the Social Phobic Disorders Severity and Change Form (
33), which yielded two continuous measures, a Social Phobic Disorders Severity score and a Social Phobic Disorders Change score. These scores are the Clinical Global Impression scores (severity and improvement, respectively) with the wording modified specifically to measure symptoms of social anxiety disorder (
33). These clinical interviews were conducted by an interviewer blind to treatment assignment.
Response was defined as an improvement score of 1 (“very much improved”) or 2 (“much improved”) on the Social Phobic Disorders Change scale; remission was defined as an improvement score of 1 or 2 on the Social Phobic Disorders Change scale and a score <30 on the Liebowitz Social Anxiety Scale.
Study Interventions
CBT.
All therapists followed the same protocol, adhering to standard treatment protocols consisting of 12 weekly 2.5-hour sessions conducted in a group format with four to six patients and two therapists per group (
34). The content of these first two sessions followed the treatment protocol outlined by Hope et al. (
35), and the remaining sessions adhered to the exposure targets and strategies described by Hofmann and Otto (
34). Sessions 3–7 emphasized exposure to feared cues (e.g., public speaking, calling a stranger, returning goods to a store) with the aim of achieving fear extinction. Session 8–12 involved a combination of cognitive restructuring and individually tailored in vivo exposure practices designed to challenge the patient’s maladaptive beliefs about the negative consequences of social mishaps (
36). In addition, patients received homework assignments between sessions to practice the techniques they learned during the sessions. All therapists were trained and supervised by senior clinicians (S.G.H., J.A.J.S., L.M.) and participated in weekly cross-site supervision (led by S.G.H. and J.A.J.S.).
Medication.
Study capsules were prepared containing either 50 mg of d-cycloserine or placebo and were administered by a nurse or a psychiatrist 1 hour before sessions 3–7. All capsules were identical in appearance to maintain the blind. The active drug capsules contained 50 mg of d-cycloserine (derived from 250 mg capsules of cycloserine [Seromycin]) and polyethylene glycol 3350 powder, while the matching placebo capsules contained only the polyethylene glycol 3350 powder. All participants were observed when they ingested the pill.
Data Analysis Plan
The primary analyses were conducted using mixed-effects regression models for continuous outcome variables (Social Phobic Disorders Severity and Liebowitz Social Anxiety Scale scores), and generalized linear mixed models (mixed-effects regression models using a logistic linking function) for dichotomous outcomes (remission and response). These analytical approaches allow the inclusion of all subjects, regardless of missing data, thereby improving statistical power and the generalizability of the results, and they are the recommended approaches to analyzing longitudinal psychiatric data (
37). Because the growth curve of the outcome measures changed markedly from treatment through follow-up, the curve was modeled as “discontinuous” (
38), allowing all growth curve parameters to change from the treatment phase to the follow-up phase. The predictors in the models were time (t), t
2, treatment condition (T), T×t and T×t
2. In addition, because of the disproportionally higher rate of males in the
d-cycloserine-augmented CBT condition, sex along with the interaction of sex and time were added as additional covariates in the model. Because initial severity is likely to be related to rate of improvement in outcomes, we also included initial severity and the interaction of initial severity with time as additional predictors of outcome to reduce variance in outcome. The growth curves during the treatment phase and the follow-up periods were linear for both of the continuous outcome measures (Social Phobic Disorders Severity and Liebowitz Social Anxiety Scale scores) but were curvilinear for the treatment phase in the analysis of the dichotomous outcomes (remission and response). Thus, the quadratic terms for the growth curve were omitted from the mixed-effects regression model analyses of Social Phobic Disorders Severity and Liebowitz Social Anxiety Scale scores and retained in the generalized linear mixed-model analyses for the treatment phase of the analysis of response and remission. Neither sex nor the interaction of sex and time were significant predictors of any of the outcomes.
Results
Treatment Attrition and Integrity
Attrition rates during the 12-week treatment phase were low and did not differ significantly between groups (10.3% for the
d-cycloserine-augmented CBT group and 15.9% for the placebo-augmented CBT group). (The reasons for attrition are listed in the flow diagram in the online
data supplement.) Similarly, attrition was low during the follow-up phase (11.5% and 11.0% for the
d-cycloserine and placebo groups, respectively). The mean number of CBT sessions attended was 10.8 (SD=2.19) in the
d-cycloserine-augmented condition and 10.6 (SD=2.14) in the placebo-augmented condition. Similarly, compliance with pill administration was high, with 78.1% of patients receiving all pill administrations (
d-cycloserine group, 75.9%; placebo group, 80.5%).
At the beginning of sessions 3–7 when the study pills were administered, patients were asked to indicate whether they believed the pill contained d-cycloserine or placebo or whether they were unable to guess. Approximately one-third to one-half of all patients (30.9%–46.2%, depending on group and session) in both conditions reported that they were unable to guess their treatment condition (all chi-square tests, n.s.). Among patients who guessed either of the two drug conditions, those who received d-cycloserine did not differ significantly from those who received placebo in their guess that they received d-cycloserine, in any of the sessions.
Baseline Severity
There were no differences between groups at baseline on Liebowitz Social Anxiety Scale score (mean=81.34 [SD=15.06] compared with mean=81.98 [SD=17.15]), Social Phobic Disorders Severity score (mean=5.33 [SD=0.83] compared with mean=5.26 [SD=0.86]) or depressive symptom severity. The median number of comorbid diagnoses in both groups was 1.
Primary Analyses: Response and Remission Rates
An intent-to-treat analysis using the last-observation-carried-forward approach revealed response rates of 79.3% in the
d-cycloserine-augmented group and 73.2% in the placebo-augmented group (
Table 2). The remission rates of these intent-to-treat analyses were 34.5% and 24.4% for the
d-cycloserine and placebo groups, respectively. The difference between groups was not statistically significant for either response or remission rate.
The follow-up analyses using traditional intent-to-treat analyses also revealed no significant differences between the d-cycloserine-augmented and placebo-augmented groups in response rates at the 1-month follow-up (74.7% and 75.6%, respectively), the 3-month follow-up (73.6% and 70.7%, respectively), or the 6-month follow-up (70.1% and 74.4%, respectively). Similarly, these analyses revealed no between-group differences for remission rates at the 1-month follow-up (33.3% and 28.0%, respectively), the 3-month follow-up (29.9% and 28.0% respectively), or the 6-month follow-up (29.9% and 28.0%, respectively).
The mixed-effects regression models revealed that at the posttreatment assessment, the
d-cycloserine-augmented group had lower mean global illness severity scores, as measured with the Social Phobic Disorders Severity score, than the placebo-augmented group (estimated parameter for treatment effect: 0.43; t=2.05, df=149, p=0.04). We also observed a statistical trend toward lower scores in the Liebowitz Social Anxiety Scale at the posttreatment assessment with
d-cycloserine compared with placebo (estimated parameter for treatment effect: 5.22; t=1.75, df=146, p=0.08). Unadjusted means and 95% confidence intervals are presented in
Table 2.
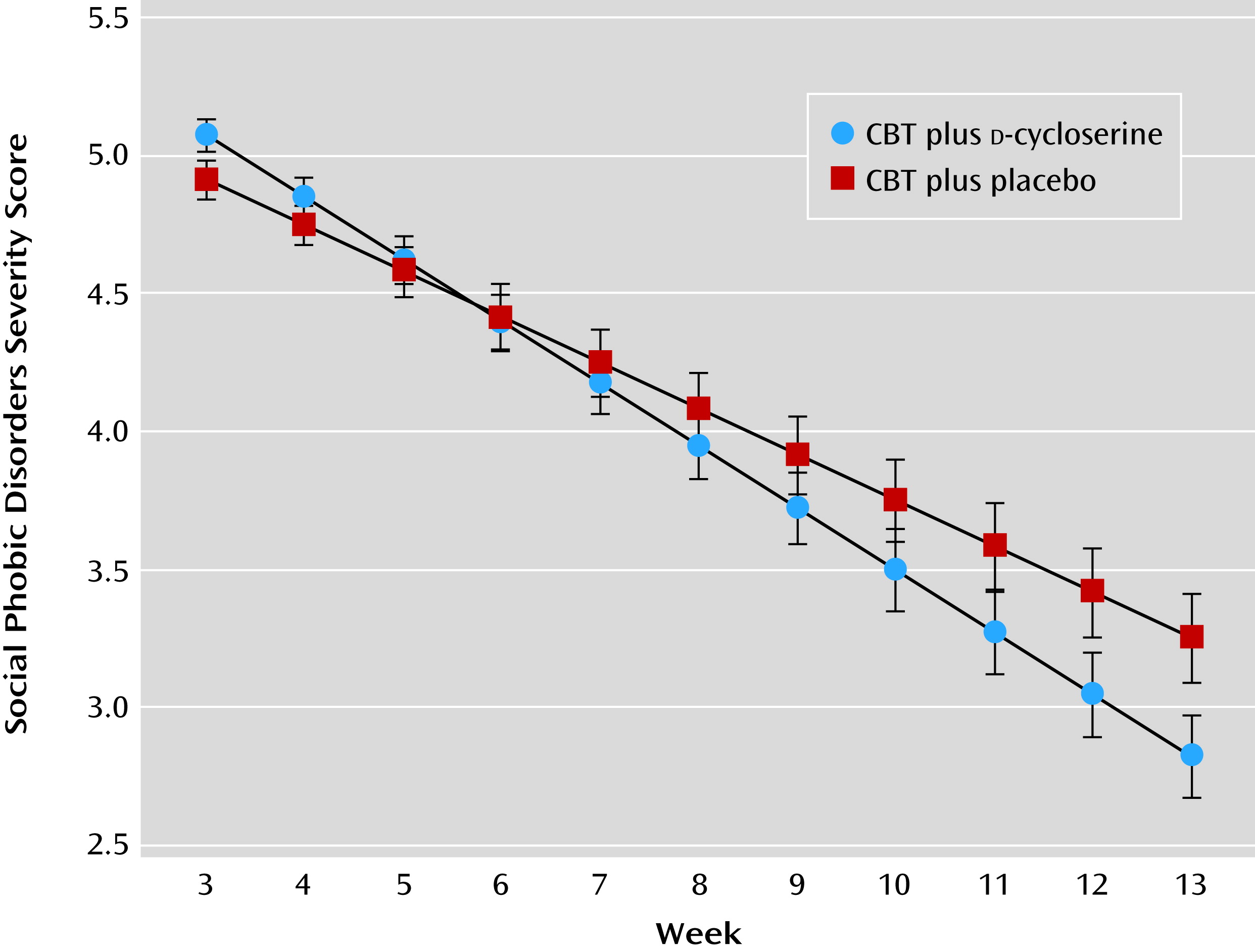
Secondary Analyses: Slope Analyses and Speed of Response
During the treatment phase, patients who received
d-cycloserine-augmented CBT exhibited a faster slope of improvement on global illness severity (Social Phobic Disorders Severity scores; estimated parameter for treatment effect: 0.06; t=2.76, df=129, p=0.006) (
Figure 1), on social anxiety symptom severity scores (Liebowitz Social Anxiety Scale; estimated parameter for treatment effect: 0.65; t=2.18, df=147, p=0.03), and on remission rates (estimated parameter for treatment effect: –0.10; t=2.59, df=1661, p=0.01) as compared with those who received placebo-augmented CBT. Compared with patients in the placebo group, those in the
d-cycloserine group showed a 33% faster rate of improvement in Social Phobic Disorders Severity scores, a 24% faster reduction in Liebowitz Social Anxiety Scale scores, and a 30% faster increase in remission rates during treatment. There was no between-group difference on slopes of change in response rates.
During the follow-up period, the mixed-effects regression model and generalized linear mixed-model analyses showed that none of the slopes of change for any of the outcome measures (Social Phobic Disorders Change scores, Liebowitz Social Anxiety Scale, response, and remission) varied as a function of condition. Patients receiving
d-cycloserine did not evidence significantly better outcomes on any of the measures at the posttreatment or follow-up assessments. Unadjusted means and 95% confidence intervals are presented in
Table 2.
Discussion
To our knowledge, this is the first large-scale randomized placebo-controlled clinical trial to evaluate
d-cycloserine as an augmentation strategy for a full course of comprehensive CBT for social anxiety disorder.
In contrast to our prediction, d-cycloserine-augmented CBT was not superior to placebo-augmented CBT in completion rate, response rate, and remission rate at the posttreatment or follow-up assessments. These findings are at odds with our earlier pilot study (20), an independent replication (21), and other small-scale proof-of-concept trials with other anxiety disorders, including panic disorder (39) and height phobia (40). Instead, our findings are in line with other studies (22, 24, 25) suggesting that d-cycloserine does not amplify the effects of CBT at major endpoints but may temporarily accelerate therapy gains.One important difference between these previous studies and the present trial is the length of treatment. In our earlier study (
20) and the subsequent replication (
21), the sessions consisted of only four 1-hour public speaking exposures. The greater number and more tailored exposure sessions in the present study may have provided less room for a memory enhancement effect of
d-cycloserine, as suggested by the response rates >70%, which is higher than what has typically been reported in studies examining traditional CBT protocols for social anxiety disorder. For example, a large-scale study that employed similar study criteria (
2) reported an intent-to-treat response rate of 50.8% after 12 weekly sessions of traditional CBT plus pill placebo. A more recent trial (
8) reported a response rate of only 47.1% after monotherapy with traditional CBT. The treatment protocol we used in the present study differed from these traditional CBT approaches primarily in the nature of the exposure practices. The protocol included in vivo social mishap exposures, which were also encouraged as homework practice exercises between sessions. This may have contributed to the relatively high response rates in both groups. Both treatments were also associated with relatively low dropout rates, which did not differ between the two treatments.
Our study provides evidence for limitations of d-cycloserine as a combination strategy with a full course of comprehensive CBT. Although patients who received d-cycloserine-augmented CBT showed a 24%–33% faster rate of improvement in symptom severity and remission rates relative to those who received placebo-augmented CBT, no between-group differences were evident in response or remission rates at the posttreatment assessment or any of the follow-up assessments. However, it is possible that a difference in dosing or dose timing of d-cycloserine might have led to different results. It is further possible that d-cycloserine is more effective for some subgroups of patients than for others, including those with a specific genotype and those who demonstrate greater within-session progress. Another limitation is the relatively stringent, although not atypical, study criteria. However, only 20% of patients (71 of 360) were excluded because of the study criteria.
In sum, this first large-scale placebo-controlled randomized trial showed that
d-cycloserine did not augment the efficacy of CBT for social anxiety disorder. However, in line with results of other studies (
14,
28),
d-cycloserine may temporarily accelerate the speed of treatment response. This acceleration may provide early treatment benefit, affording a potential role for
d-cycloserine in reducing patient distress during exposure treatments. This may enhance the acceptability of treatment and limit avoidance tendencies of patients with anxiety disorders that can hinder the success of CBT. This study tested the use of
d-cycloserine as a routine augmentation strategy for a full course of CBT for social anxiety disorder; we recommend that future studies examine whether
d-cycloserine augmentation might be indicated for any specific conditions or patient characteristics.
Acknowledgments
The authors thank Stephen Wisniewski, Ph.D., for providing unpaid statistical consultation during the design development and Richard G. Heimberg, Ph.D., for providing a 1-day training and consultation workshop in cognitive-behavioral group therapy prior to participant enrollment (he received compensation for this contribution). The authors also thank Ashley Witt, B.A., and Shelley Capozzoli, B.A., at Boston University for their help with the data entry and management. The authors also thank the following individuals for serving as therapists or clinical assessors in the study: at Boston University, Allison Applebaum, Ph.D., Anu Asnaani, M.A., Jacqueline Bullis, M.A., Cassidy A. Gutner, M.A., John A. Richey, Ph.D., Alice T. Sawyer, M.A., and Maria Steenkamp, Ph.D.; at Southern Methodist University, Lindsey DeBoer, M.A., Deborah Corbitt-Shindler, M.A., Katherine Croft, M.A., Catherine Dodson, M.A., Pamela Handelsman, B.A., Grant Holland, M.A., Kristin Julian, M.A., Erica Simon, M.A., Anne Miller, M.A., and Matthew Leahy, Ph.D.; and at Massachusetts General Hospital, Ryan Jacoby, B.A., Meghan Keogh, Ph.D., Libby Marks, B.A., Laura Morris, B.A., Don Robinaugh, M.A., and Sharon Sung, Ph.D.