Since Kraepelin’s initial distinction (
1), schizophrenia and bipolar disorder have been viewed as separate clinical entities with distinct clinical courses and outcomes. However, significant overlaps exist in symptoms (
2), alterations in cognition (
3), brain structure (
4), brain functioning, and disease risk genes, especially between schizophrenia and psychotic bipolar disorder (
4). Both disorders are heritable; large meta-analytic linkage studies based on clinical schizophrenia and bipolar phenotypes have shown several overlapping genetic risk loci (
5). Schizophrenia and affective psychoses co-occur within kindreds, suggesting shared familial risk and consistent with shared genes that confer risk for both illnesses, most convincingly for schizophrenia and psychotic bipolar disorder. Thus, some illness risk genes and associated brain processes are shared by schizophrenia and psychotic bipolar disorder, while others are likely disease-specific. Such deficits also occur in unaffected relatives of probands, in whom illness risk genes are likely overrepresented (
6) in comparison to the population at large. Such research in schizophrenia has identified several functional and anatomical indicators of disease risk, known as endophenotypes. Endophenotypes are heritable, quantifiable, trait-related, illness-associated biological features that cosegregate with disease in families, are overrepresented in the unaffected relatives compared with the general population (
7), and are conceived of as situated between genes and phenomenology.
The dysconnectivity model of schizophrenia (
8) posits that several brain circuits interact abnormally to generate the schizophrenia phenotype; this proposal is supported by reported functional connectivity deficits (
9). While empirical support for dysconnectivity is strongest at the functional level, it is plausible that connection problems originate in disrupted white matter connections. Integrity of those connections is usually measured by using estimates of brain tissue water diffusion obtained with diffusion tensor imaging (DTI), mainly fractional anisotropy. In schizophrenia, differences (
10) in white matter structure and integrity have been observed both as a global measure and in specific structures. The relationship between altered structural and functional measures has been demonstrated previously (
11). Analogous abnormalities may be present among unaffected relatives of persons with schizophrenia (
9), suggesting that brain dysconnectivity may be a schizophrenia endophenotype (
12). Similar but less consistent deficits are reported for bipolar disorder (
13).
Confirming other findings (
9), we previously reported functional network differences assessed with resting-state functional magnetic resonance imaging (fMRI) (
14). In a large multicenter study, we have now investigated whether white matter connectivity is similarly impaired in probands with schizophrenia, probands with psychotic bipolar disorder, and their first-degree relatives, to address the endophenotypic status of any such deviations. Given the known associations between cluster A and B personality traits and risk for schizophrenia (
15) and psychotic bipolar disorder (
16), we also examined abnormalities in relatives with cluster A or B personality traits, the relationship between fractional anisotropy and a measure of the continuum between schizophrenia and affective disorder (
2), and the heritability of observed abnormalities. As the validity of the diagnostic status of schizoaffective disorder is controversial (
17), we decided not to analyze probands with schizoaffective disorder and their relatives as separate groups. These 35 probands were classified as having either bipolar disorder (schizoaffective disorder, manic) or schizophrenia (schizoaffective disorder, depressed), as suggested elsewhere (
2,
5).
Results
Differences Between Diagnostic Groups in Probands and Relatives
We first analyzed global mean fractional anisotropy averaged over the whole white matter skeleton. Fractional anisotropy in both proband groups was significantly lower (p<0.001) than in the healthy comparison subjects (t=4.82, df=227, for schizophrenia; t=3.69, df=184, for psychotic bipolar disorder; effect size: d=0.64 and 0.54, respectively), with no differences between proband groups (effect size: d=0.04). Fractional anisotropy was lower in 29 regions (of 76 analyzed) in probands (
Table 2); none differed significantly between schizophrenia and psychotic bipolar disorder. For all regions, effect sizes for the differences between proband groups were less than 0.2. The global fractional anisotropy average showed significantly higher (p<0.01) variance in the bipolar probands than in either the schizophrenia probands or the comparison subjects and nonsignificantly higher variance in the relatives of the bipolar probands (p<0.06).
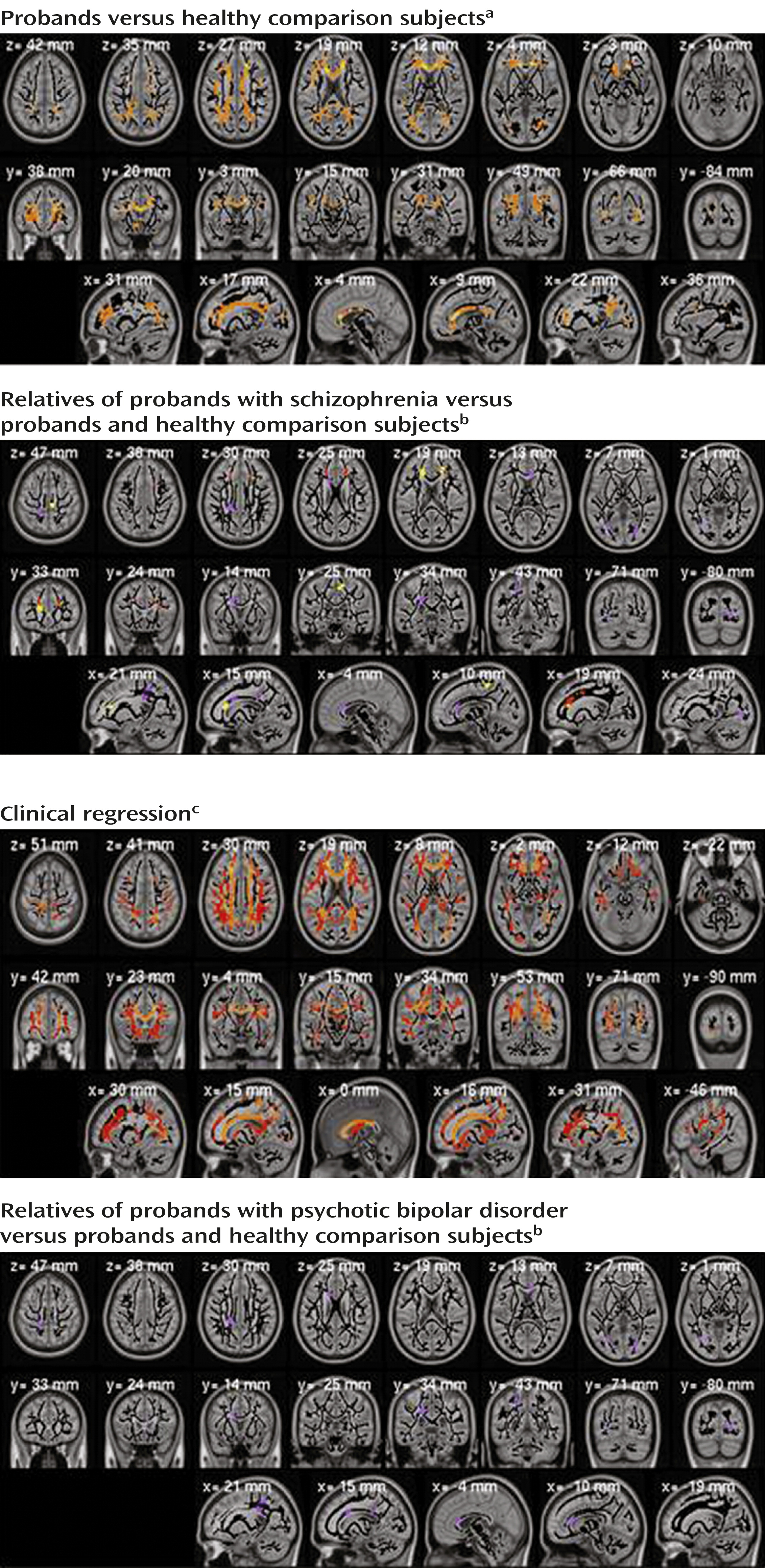
Figure 1 (top left) shows the distribution of differences between the healthy comparison subjects and the clinical groups (p<0.01, corrected). Many regions revealed fractional anisotropy differences for schizophrenia but not psychotic bipolar disorder. With the exception of one cluster, all voxels showing differences in psychotic bipolar disorder also showed differences in schizophrenia. None of the fractional anisotropy values was higher in a clinical group than in the comparison subjects.
Among the relatives, the whole-brain average for fractional anisotropy was lower in the schizophrenia relatives than in the healthy comparison subjects (t=2.62, df=221, p<0.01, d=0.35). Two anatomically defined regions (left anterior corona radiata and superior right corona radiata) differed significantly (p<0.05 corrected) between the schizophrenia relatives and comparison subjects (
Table 2). Table S1 and Figures 1S and 2S in the online
data supplement shows results of the focused post hoc analyses of the regions of interest. In the secondary analyses, fractional anisotropy in the schizophrenia relatives was significantly lower than in the healthy comparison subjects in 10 regions and higher than in the schizophrenia probands in 11 regions.
Figure 1 (top right) shows the spatial distribution of those differences. In many regions, the relatives in the schizophrenia group closely resembled the probands. Only the corpus callosum genu in the schizophrenia relatives differed significantly from that region in both the schizophrenia probands and the healthy comparison subjects.
The relatives of the probands with psychotic bipolar disorder showed fewer significant effects, with only one region where their fractional anisotropy was lower than that of the healthy comparison subjects (superior aspect of the left posterior corona radiata) and five regions where their values were higher than those of the bipolar probands. No analyzed region showed significant differences between schizophrenia and psychotic bipolar disorder for either the probands or the relatives.
Most of the 29 regions differing between probands and healthy comparison subjects also manifested a significant clinical regression effect for schizophrenia; in psychotic bipolar disorder this effect was restricted to the corpus callosum genu (
Table 2). Similarly, in voxel-based analysis (
Figure 1, bottom left) most regions showing differences between probands and comparison subjects manifested similar clinical regression effects. As with these clinical contrasts, correlations between fractional anisotropy and clinical status were higher in schizophrenia than in psychotic bipolar disorder.
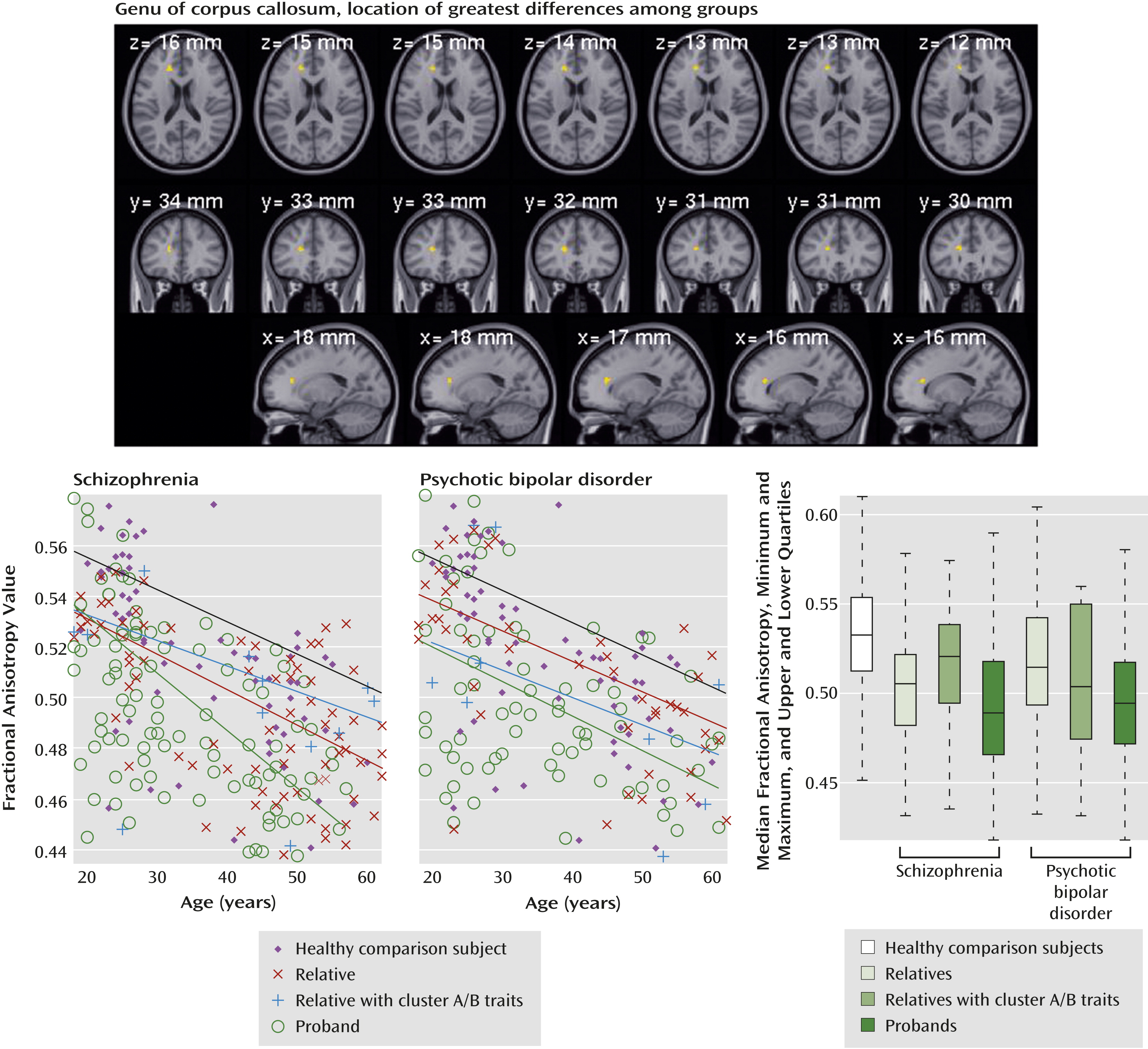
Fractional anisotropy values are displayed in
Figure 2 for the anatomically defined genu of the corpus callosum, the region showing the strongest differences among groups.
To study effects of psychosis common to both diseases, we analyzed group differences (probands and relatives versus comparison subjects) and clinical regression correlations with the data for schizophrenia and psychotic bipolar disorder combined. The region-of-interest analysis of clinical regression and the analysis of probands versus comparison subjects showed that all regions showing significant differences for schizophrenia (
Table 2) also showed lower values in the joint group analysis. In the comparisons of relatives and comparison subjects, only one region (left anterior corona radiata) showed significant difference in the combined analysis.
Cluster A or B Traits in Relatives
Few relatives met our criteria for cluster A or B personality disorders, so their comparisons with relatives lacking those personality characteristics had lower statistical power. Nevertheless, in the analysis comparing cluster A relatives to other relatives, two regions (
Table 2) showed significant (p<0.05) differences for the relatives of probands with schizophrenia, and one region showed a difference for the relatives of the bipolar probands. Both the corpus callosum genu and right posterior thalamic radiation skeleton showed significant stepwise progressions of fractional anisotropy values from the healthy comparison subjects to relatives to cluster A relatives to schizophrenia probands, with the cluster A relatives resembling the probands. Similar trends were seen in other regions where probands differed from comparison subjects. No such differences were observed for relatives with cluster B traits.
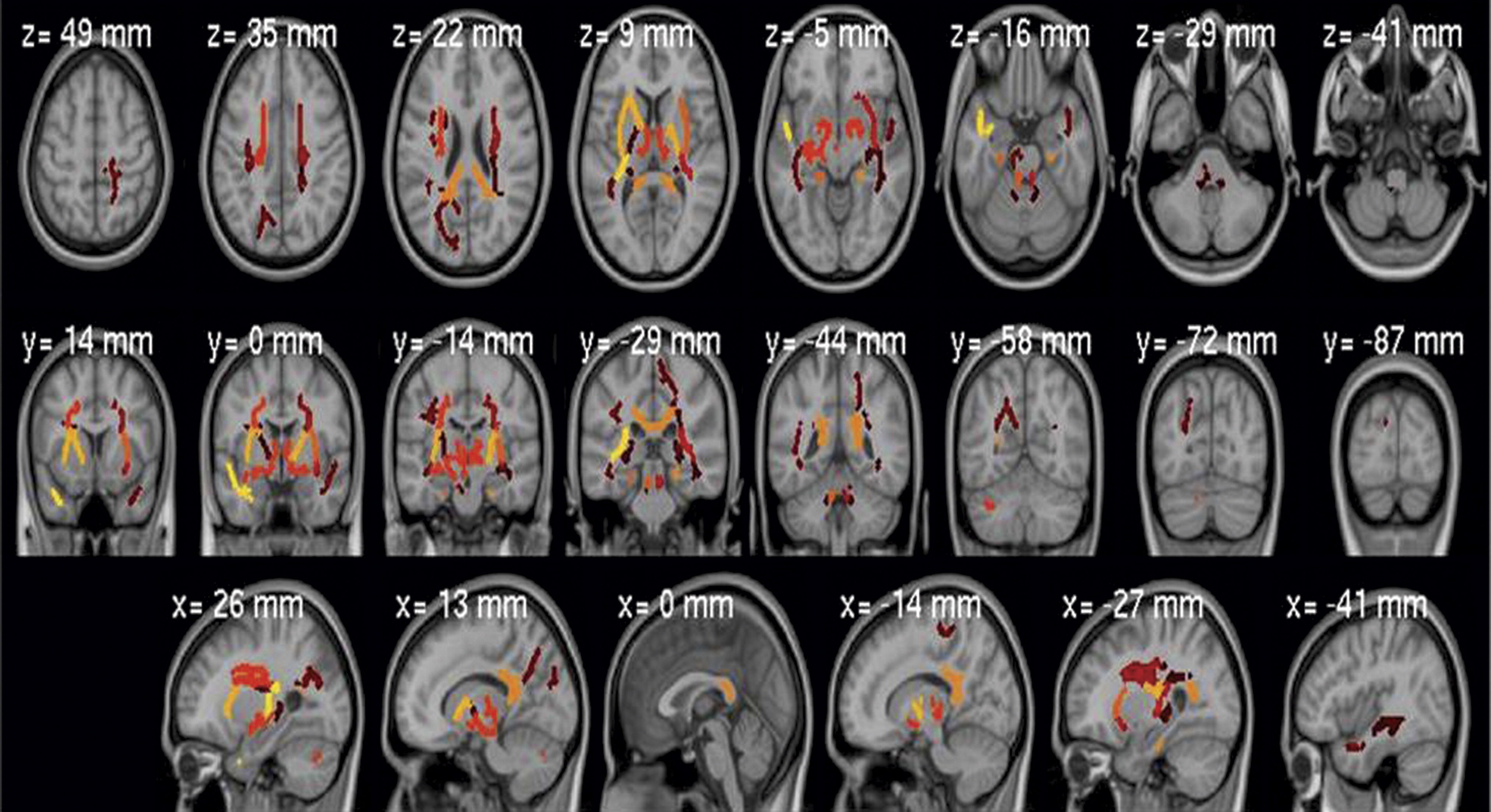
Heritability
Whole-brain mean fractional anisotropy was highly heritable (h
2=0.45, p<0.001), as was fractional anisotropy for many anatomically defined regions (
Table 2,
Figure 3), not restricted to those showing between-group effects (see Table S1 in the online
data supplement).
Correlation With Schizo-Bipolar Scale Scores
Analysis of anatomically defined regions of interest showed a weak association between scores on the Schizo-Bipolar Scale and fractional anisotropy values (t=2.11, df=181, p<0.05, uncorrected) in the left superior longitudinal fasciculus, peaking at MNI coordinates –33, –19, 41 (see Figure 3S in the online
data supplement).
Demographic Measures
While the general linear model approach removes effects of confounding variables, interactions among sex, race or ethnicity, age, and diagnosis are relevant for understanding pathologic and clinical applications. Separate general linear model analyses were performed to examine those interactions. Age was the most important confounding factor; fractional anisotropy values decreased significantly with age in all groups and regions of interest. For the global whole-brain average, age interacted with disorder. The interaction was negative in the probands with psychotic bipolar disorder and their relatives (less decrease with age than in comparison subjects). In schizophrenia it was positive for the whole-brain average and for the superior posterior corona radiata, where fractional anisotropy values decreased more steeply with age than they did among the healthy comparison subjects.
In the analysis of whole-brain mean fractional anisotropy, sex and race showed significant differences, with men and European Americans showing higher values. The effects of sex and race on global fractional anisotropy did not interact with clinical diagnosis. At the regional level, sex showed an interaction (t>3.0, p<0.01) in five regions in the comparison of bipolar probands and healthy comparison subjects: the corpus callosum body (MNI coordinates 13, 3, 33), right and left anterior limbs of the internal capsule (MNI coordinates 23, 6, 20 and –12, 14, 17), right anterior corona radiata (MNI coordinates 23, 27, 5), and right inferior fronto-occipital fasciculus (MNI coordinates 29, 14, –7). The direction of the interaction was such that in women the decrease of fractional anisotropy in the bipolar probands was larger than in men.
Other DTI Measures
We also analyzed radial diffusivity, mean diffusivity, and axial diffusivity. They showed no significant differences at the threshold used for fractional anisotropy. We additionally investigated data pooled in all voxels showing significant changes in fractional anisotropy. As expected, fractional anisotropy decreases were accompanied by increases in all three diffusivity measures. A significant (p<0.01) difference was observed; differences in radial diffusivity were larger than those for mean and axial diffusivity in both the schizophrenia and bipolar probands, suggesting that demyelination (not axonal injury) causes the observed white matter differences.
Scanner Effects and Reproducibility
After we adjusted for age, mean whole-brain fractional anisotropy did not differ between scanners. Site showed no interaction with clinical diagnosis, and the spatial map of scanner effects did not correlate with clinical effect maps. Quantifying between-scanner reproducibility as a stand-alone measure is difficult. To estimate it, we ran general linear model analyses for each scanner separately and calculated general linear model contrast maps for each site. To quantify their agreement, we developed a randomization procedure in which data from both scanners were randomly split into groups the size of the scanned subjects at each site; the general linear model was run for each data set separately, and the degrees of reproducibility in the subsamples were compared. The between-scanner correlation (r) values were 0.60 for age, 0.12–0.18 for the main contrast of probands and healthy comparison subjects, 0.05–0.10 for the main contrast of relatives and comparison subjects, and 0.06–0.12 for the contrast of schizophrenia and bipolar probands. In randomization procedures, real comparisons fell between 25% (schizophrenia probands versus comparison subjects) and 6% (relatives of bipolar probands versus comparison subjects) of randomized splits. Thus, the between-scanner data agreement was not significantly worse (p<0.05) than would be expected from two data sets derived from the same scanner. General linear model analyses performed separately for each scanner showed the same, but less significant, findings than in the combined analysis.
Medication
We compared probands receiving a particular medication class with all other probands. Patients with psychotic bipolar disorder who took second-generation antipsychotic drugs showed significantly higher fractional anisotropy in the corpus callosum genu and body (t=2.93, df=80, p<0.01 uncorrected) than did the remaining bipolar probands. For schizophrenia, a significant effect was observed in the corpus callosum splenium for anticonvulsant mood stabilizers (t=–3.31, df=123, p<0.01), such that probands with schizophrenia who were taking these medications had lower fractional anisotropy values than other schizophrenia probands.
Discussion
We compared fractional anisotropy values of healthy comparison subjects to those of a large group of probands with schizophrenia or psychotic bipolar disorder and their first-degree relatives. After identifying between-group differences, we assessed whether deviations observed in probands might qualify as candidate endophenotypes (i.e., were also observed in their relatives). Our results show that despite scanner or site differences, DTI studies from multiple sites can be successfully combined to achieve increased statistical power for detecting differences between clinical groups.
The white matter abnormalities in schizophrenia and psychotic bipolar disorder were similar in spatial distribution. The corpus callosum genu and body showed the most significant deficits in fractional anisotropy in both proband groups, in agreement with previous reports (
32). Like others (
23), we found no significant differences between the probands with schizophrenia and those with psychotic bipolar disorder. The close agreement between the spatial distributions of maps of fractional anisotropy differences, similar magnitudes of those differences, and higher variance in the bipolar probands suggest that this might result, in part, from greater heterogeneity of white matter abnormalities in bipolar disorder (
4,
25) than in schizophrenia.
In comparison to findings based on diagnostic categories, ratings on the dimensional Schizo-Bipolar Scale (
2) revealed a modest correlation with fractional anisotropy in the left superior longitudinal fasciculus, suggesting that this region distinguishes schizophrenia and psychotic bipolar disorder. This fasciculus, connecting Broca’s and Wernicke’s areas, has been reported as abnormal in both schizophrenia (
2) and bipolar disorder (
33). As no differences were found between the schizophrenia and bipolar probands in fractional anisotropy, scores on the Schizo-Bipolar Scale may capture subtle differences between those diseases better than clinical diagnosis.
Many abnormalities present among probands were observed, in attenuated form, in first-degree relatives. Indeed, there was a linear, stepwise progression from healthy comparison subjects to noncluster relatives to cluster A relatives to probands. The schizophrenia relatives generally showed fractional anisotropy deviations similar to, but less significant than, those seen in the schizophrenia probands. Fractional anisotropy decreased with age in all groups, an effect that was exaggerated in schizophrenia but not psychotic bipolar disorder, suggesting more pronounced progressive white matter abnormalities in schizophrenia. In psychotic bipolar disorder, fractional anisotropy decreases may occur early and not progress thereafter.
The subgroups of relatives with cluster A or B symptoms resembled the probands more than the other relatives did. This effect was most pronounced in the corpus callosum genu and posterior thalamic radiation skeleton of the schizophrenia relatives, but it was also seen in the bipolar probands’ relatives in the corpus callosum genu. While the schizophrenia and bipolar probands showed similar differences from the comparison subjects, the relatives showed weaker, more diverse differences. This was illustrated by the analysis that combined data from the schizophrenia and bipolar relatives and compared them to the healthy subjects, which showed less significant effects than analyses in each group of relatives separately. Thus, the differences in relatives were smaller in effect size and more disease specific. The relatives’ fractional anisotropy values fell between those of the healthy comparison subjects and the probands, but abnormal regions differed for each disorder. Overall, our findings point to similarity in the distributions of white matter deficits in schizophrenia and bipolar probands, with similar but attenuated abnormalities observed in their relatives. This suggests that genetic risk factors present in the relatives differ from those in the probands, perhaps indicating that protective genetic variants are exerting effects visible at a DTI level.
Current medications showed different effects in the clinical groups. Interpretation of those effects is difficult, as the study design did not allow us to differentiate among premedication patterns of symptoms or severity, prescription bias, and medication effects. Comparisons of the medication groups seemed to exclude medications as the main cause of lower fractional anisotropy. Analysis of the sex and race interactions showed lower fractional anisotropy values in women and in African American subjects, with sex-by-diagnosis interactions stronger for psychotic bipolar disorder.
Fractional anisotropy values decreased significantly with age in all groups and most brain regions. In the whole brain and one region, this finding interacted with diagnosis for schizophrenia: age-related fractional anisotropy decreases were significantly greater in the schizophrenia probands, consistent with findings in other studies (
34). This may support the hypothesis (
35) that schizophrenia is a progressive brain disorder, perhaps related to accelerated aging (
36) and/or medication effects. The probands with psychotic bipolar disorder and their relatives showed opposite effects: the differences from the comparison subjects were strongest for younger probands and relatives, disappearing with increasing age. This may explain why the present study did not replicate the widespread fractional anisotropy decreases in relatives of bipolar probands that were reported earlier (
28) in younger subjects. Our global analysis showed that the age-related fractional anisotropy decrease was smaller for the bipolar probands and their relatives than for schizophrenia probands and healthy subjects. In addition, limiting the group to younger subjects yielded a stronger difference in fractional anisotropy among the relatives of bipolar probands. Thus, the fractional anisotropy differences between relatives of bipolar probands and healthy subjects may have been driven by the proportion of younger subjects in this study group. Perhaps the younger relatives of the bipolar probands included subjects who will later develop clinical symptoms, or perhaps endophenotypic white matter features in relatives of individuals with psychotic bipolar disorder manifest early and do not progress with age.
Table 2 lists previous fractional anisotropy reports that agree with our findings. We consulted three recent meta-analyses (
4,
23,
37) of fractional anisotropy findings for schizophrenia and eight for bipolar disorder (
23,
25–
27,
29,
30,
38,
39). Of 18 coordinates reported in the schizophrenia reviews, 15 fell within regions found in our study. Of 21 coordinates reported in studies of bipolar disorder, 10 were located within regions we detected. Major regions reported in these reviews but not identified in our study included the left posterior retrolenticular internal capsule (
23), left superior corona radiata (
23), left cingulum (
25), right fornix (
23), right superior (
23) and inferior (
40) fronto-occipital fasciculus, and left superior anterior corona radiata (
27).
Possible study limitations should be noted. The large number of subjects was obtained at the cost of combining studies from two different scanners and slightly different pulse sequences. Between-scanner effects were present but were not correlated with clinical contrast results and were successfully removed in the general linear model as confirmed by randomized comparison of maps obtained from each scanner. The study design did not allow full analysis of medication effects. The study groups were not precisely matched demographically, and so age and sex effects had to be accounted for by the model, reducing power. Neither disease severity nor patient medication response was assessed or included in the analyses.
Overall, these findings from a large multisite study show strong agreement with existing reports of differences in fractional anisotropy between healthy comparison subjects and probands with schizophrenia or bipolar disorder. Abnormalities in fractional anisotropy were remarkably similar in spatial distribution in the two diseases. Relatives showed similar, attenuated changes in some affected regions, most pronounced in the relatives of schizophrenia probands. Differences in the relatives of the bipolar probands were limited to younger subjects and were not significant. The endophenotypic importance of fractional anisotropy for psychosis is further supported by heritability. Fractional anisotropy was highly heritable, both for the whole-brain average and for many regions, not only those showing differences between clinical groups. Although fractional anisotropy measurements currently cannot be used clinically to diagnose psychosis, especially in individual subjects, better understanding of the relationship between white matter deficits and clinical symptoms will likely translate to improved subtyping, gene discovery, and improved future drug trials and/or other interventions. A logical next step is to combine DTI connectivity measures with functional MRI resting-state connectivity, as we did previously (
41).