Despite being the best predictor of functional outcome and quality of life in schizophrenia (
1), cognitive dysfunction remains the most poorly treated symptom. The illness is associated with deficits in multiple cognitive domains, including selective and sustained attention, working memory, episodic memory, processing speed, executive function, and social cognition (
2). Because of the lack of effective treatments for these deficits, schizophrenia often continues to exact a devastating toll, with 80% of patients remaining unemployed and less than 30% living independently (
3).
In order for therapeutic development for cognitive deficits in schizophrenia to move forward, biomarkers must be identified that can be used to determine whether therapeutic candidates elicit the targeted biological effects. In neuropsychiatric research, a biomarker is an indicator of neuronal function, hypothesized to be related to disease mechanisms, that can serve as an immediate and objective measure of the biological effects of therapeutic candidates. An effective biomarker should also be related to clinical symptoms of the illness, such that it can be used to track illness severity as a new treatment is evaluated.
Functional MRI (fMRI) and positron emission tomography studies have demonstrated abnormalities in hippocampal response in patients compared with healthy subjects. A prevailing theme of these findings is hyperactivity during tasks that require minimal or no cognitive load, such as fixation on a point (
14), smooth-pursuit eye movements (
15), passive viewing of fearful faces (
16), and passive listening to noise (
17,
18). Intrinsic hyperactivity may contribute to the inability of the region to be recruited during cognitive tasks in which it is thought to be required, such as image encoding (
19) and verbal encoding (
20). Thus, it is possible that intrinsic hyperactivity contributes to cognitive dysfunction by decreasing the dynamic range over which activity in the region can be modified according to task demands.
Despite the hypothesized role of hippocampal activity in cognitive dysfunction, no study has yet examined the relationship between intrinsic hippocampal activity and cognition in schizophrenia using a comprehensive cognitive battery. The MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) Consensus Cognitive Battery (MCCB) was created under an initiative from the National Institute of Mental Health (NIMH) to “support the development of pharmacological agents for improving the neurocognitive impairments that are a core feature of schizophrenia” by providing a “reliable and valid assessment of cognition at the level of all cognitive domains” (
2,
21). The MCCB includes seven cognitive domains: speed of processing; attention and vigilance; working memory; verbal learning; visual learning; reasoning and problem solving; and social cognition. In addition to domain index scores, a composite score is calculated based on the index scores for all domains. Despite the battery’s comprehensive nature, high test-retest reliability, predictive value, relationship to functional outcome, and utility in clinical trials (
2,
21,
22), to our knowledge no study has reported an association between MCCB scores and brain function in schizophrenia. Our goals in the present study were to test the hypotheses that 1) relative to healthy subjects, patients with schizophrenia have greater intrinsic “resting state” hippocampal activity, and 2) this activity is inversely related to cognitive ability as assessed by the MCCB. Findings in support of these hypotheses would suggest that hippocampal activity may be useful as a biomarker for therapeutic development.
Discussion
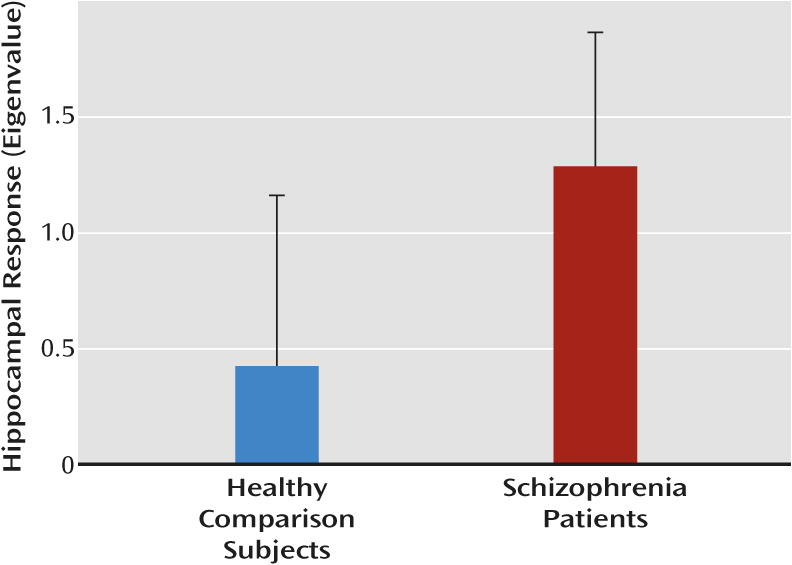
Both study hypotheses—that patients would show greater intrinsic hippocampal activity than healthy subjects and that this activity would be inversely related to cognitive function—were supported. The finding of greater resting hippocampal response is consistent with results of previous studies that have used different but conceptually related techniques, including resting cerebral blood flow and volume (
9–
13). The present findings may also be consistent with studies showing elevated hippocampal responses in patients during simple or entirely passive sensory processing (
14–
18). The observed hyperactivity both supports models of hippocampal dysfunction in schizophrenia (
37) and increases the appeal of this measure as a potential biomarker.
To our knowledge, this is the first study to report a significant association between intrinsic hippocampal activity and a broad-spectrum measure of cognition in schizophrenia. Beginning with postmortem studies in the 1970s (e.g., reference
38), investigators have repeatedly observed abnormalities in hippocampal structure and function in schizophrenia, with the most recent studies reporting increased general metabolism or activity in the region (
9,
12,
13,
39). The cognitive implications of hippocampal dysfunction have largely remained unknown, however, likely because of the lack of standardized methods for examining cognitive function in schizophrenia.
The MCCB was developed in accordance with an NIMH initiative to provide a standardized comprehensive battery of cognitive tests for use in therapeutic development. However, few studies have found associations between neuroimaging-based measures of hippocampal function and global cognition in schizophrenia, although studies have reported correlations with specific cognitive tests (e.g., reference
40). Perhaps the most comparable recent study was by Toulopoulou et al. (
41), who reported an association between IQ and right hippocampal volume across a large sample of schizophrenia patients and first-degree relatives. The results of the present study suggest that a therapeutic treatment that reduces intrinsic hippocampal activity may improve cognition in schizophrenia. Indeed, the α7 receptor partial agonists tropisetron and DMXB-A (3-[2,4-dimethoxybenzylidine] anabaseine), which have shown promising effects on cognition in early trials (
42,
43), are proposed to modulate inhibitory neurotransmission through activation of nicotinic receptors on GABA-ergic interneurons in the hippocampus (
44).
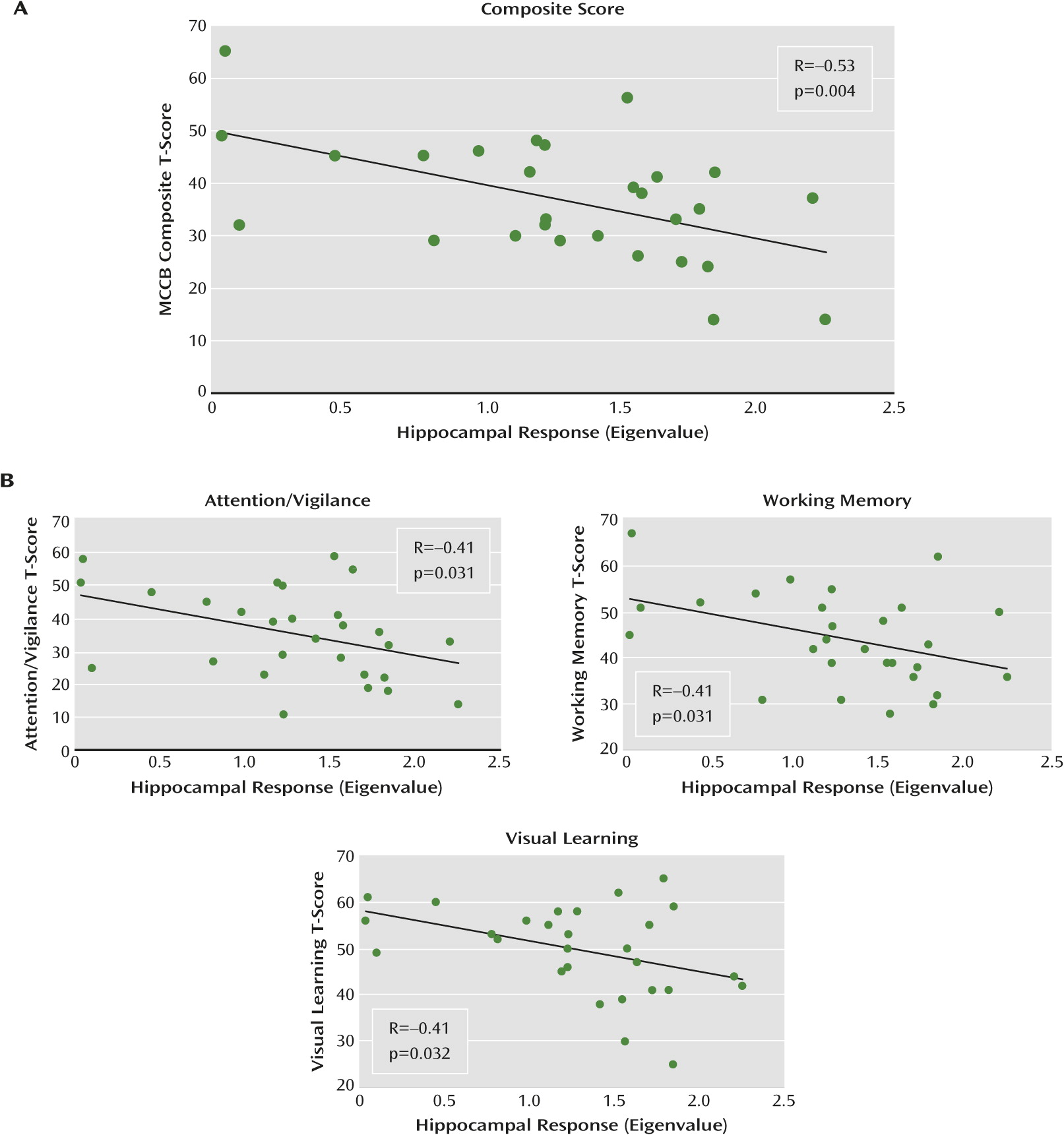
The relationship we observed between high activity and low composite MCCB score was driven by negative correlations between activity and visual learning, attention/vigilance, and working memory, which suggests that these cognitive processes may be particularly affected by hippocampal dysfunction. Although the mechanisms involved are not clear, it is possible that increased activity at “rest” reduces the region’s ability to be recruited according to task demands, resulting in impaired performance.
Multiple lines of evidence suggest that the hippocampus plays an important role in the MCCB domains driving the observed effect. For visual learning, studies have demonstrated recruitment of the right hippocampus in spatial and visual learning tasks (e.g., reference
45). In the context of attention/vigilance, the cholinergic projection from the septal area to the hippocampus is essential for selective attention (
46). Furthermore, the hippocampus exhibits sensory information processing properties, in which it is involved in the suppression of responses to repetitive stimulation (
47). As a result, it may contribute to selective attention by filtering out irrelevant information. This process is altered in schizophrenia, leading to hyperactivity in the region during the presence of distracting or unimportant noise (
17). This hippocampal hyperresponsiveness to distracting noise has been shown to be related to patients’ inability to appropriately engage cortical networks subserving selective attention (
48). In regard to working memory, hippocampal recruitment has been proposed as a secondary mechanism by which information can be actively maintained in the brain, specifically when working memory capacity in other areas (e.g., the prefrontal cortex) is exceeded (
49). A previous working memory study (
50) found increased hippocampal activity in patients relative to controls under conditions of moderate load as well as impaired performance, suggesting that functional pathology of the area may contribute to impairment of working memory in schizophrenia.
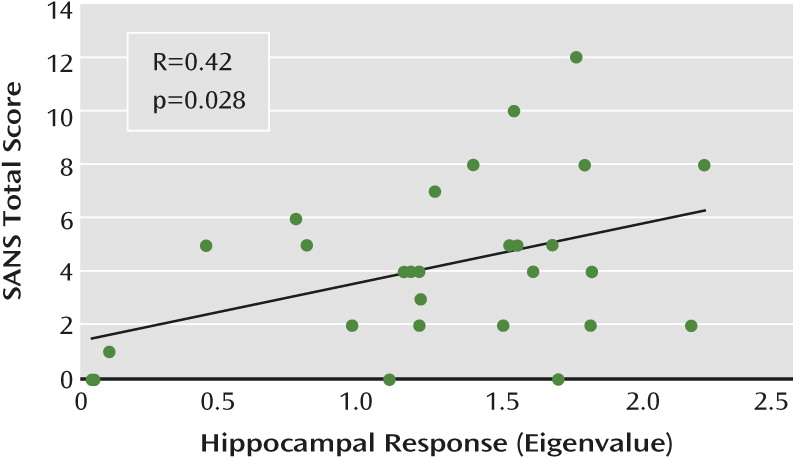
Both intrinsic hippocampal activity and MCCB scores were associated with negative symptoms. The observed relationship between cognitive dysfunction and negative symptoms is conceptually in agreement with numerous studies demonstrating stronger relationships between cognition and negative symptoms compared with cognition and positive symptoms (see reference
51). Relative to positive symptoms, cognitive and negative symptoms are more stable over time, are a better predictor of functional outcomes, and are less responsive to medication (
1,
51). Given that hippocampal dysfunction, cognitive impairment, and negative symptoms are often present before the first episode of psychosis (
12,
52), it is possible to speculate that these phenotypes may constitute core traits of the disorder that may reflect neuropathological status more accurately than do positive symptoms.
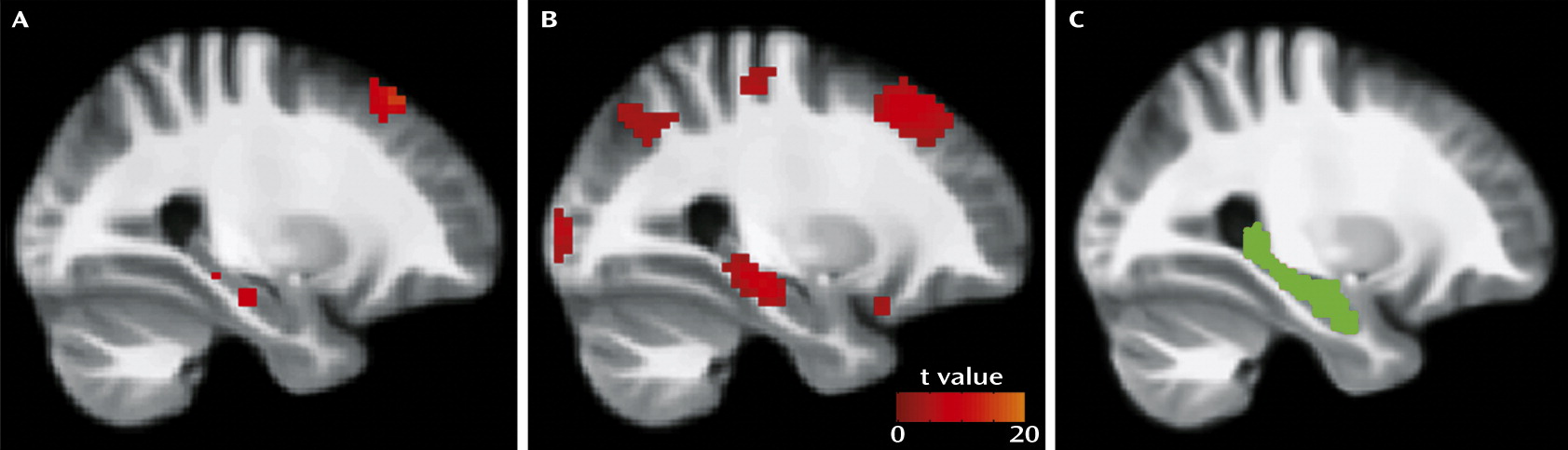
Our findings in this study were lateralized to the right. The neurobiological basis for this finding is unclear, as there is no strong evidence to suggest that hippocampal pathology is specific to either hemisphere in schizophrenia. It is unlikely that the lateralized effects were due to a floor or ceiling effect only in the left hippocampus, as no significant difference in activity was observed between the left and right hippocampi. It is possible that the lateralization we observed is a reflection of the type of MCCB tasks with which significant associations were demonstrated. For example, previous studies have found preferential involvement of the right hippocampus in visual learning and memory tasks (
45,
53). Patients with schizophrenia also demonstrate a loss of functional connectivity between the right hippocampus and the left prefrontal cortex during working memory (
54). Given that factor analyses suggest that the processing speed, attention/vigilance, working memory, and visual learning domains most strongly predict composite MCCB score (
55), the relationships between lateralized hippocampal activity and cognitive function in these domains in schizophrenia require further investigation.
Our findings should be considered in the context of the study’s limitations. In order to fully evaluate intrinsic hippocampal activity as a biomarker, its reliability must be established by studying subjects at additional time points, which ideally would be spaced sufficiently far apart for the tracking of changes in symptoms during disease progression. Another limitation is that the sample patient population in this study exhibited less impairment on the MCCB than did samples in other studies (e.g., reference
56). Additional research is needed to determine whether the observed differences in hippocampal activity and associations with MCCB scores hold true for populations with greater cognitive impairment. Finally, it is unclear whether hippocampal hyperactivity and its association with cognitive function are specific to schizophrenia. Studies examining MCCB performance and hippocampal activity in healthy subjects and other patient populations (e.g., patients with bipolar disorder) are needed to examine this possibility. Indeed, previous work has demonstrated that patients with poor working memory performance exhibit reduced right hippocampal gray matter volume compared with healthy subjects, whereas intact performers do not (
57), which is suggestive of a complex interaction between cognitive performance, hippocampal pathology, and disease state in schizophrenia.