Social impairments are a central feature of schizophrenia (
1), ranging from social withdrawal to misperceiving the intentions of others, with detrimental consequences for interpersonal relationships, quality of life, and functional outcomes. Moreover, social impairments are highly and uniquely predictive of conversion to psychosis in at-risk youths (
2). Despite an increasing focus on understanding and treating these impairments, unraveling the faulty mental operations underlying social interactions in schizophrenia has proven difficult, arguably because currently available measures and tasks of social cognition are inadequate for isolating specific mechanisms.
Action imitation, the process by which one observes and replicates the actions of others, is one such building block of social cognition. Imitation ability is present from infancy (
4) and is vital for nonverbal learning. Since social learning is almost entirely implicit and nonverbal in nature, imitation impairments can be expected to disproportionately affect social behavior. Imitation in the form of social mirroring facilitates communicative exchanges (
5), and one hypothesized route toward understanding the minds of others is via covert imitative processes (
6). That is, I understand your intentions and feelings by simulating my own experience to the same circumstances. Accordingly, imitation is considered the root of the ability to interpret the minds of others.
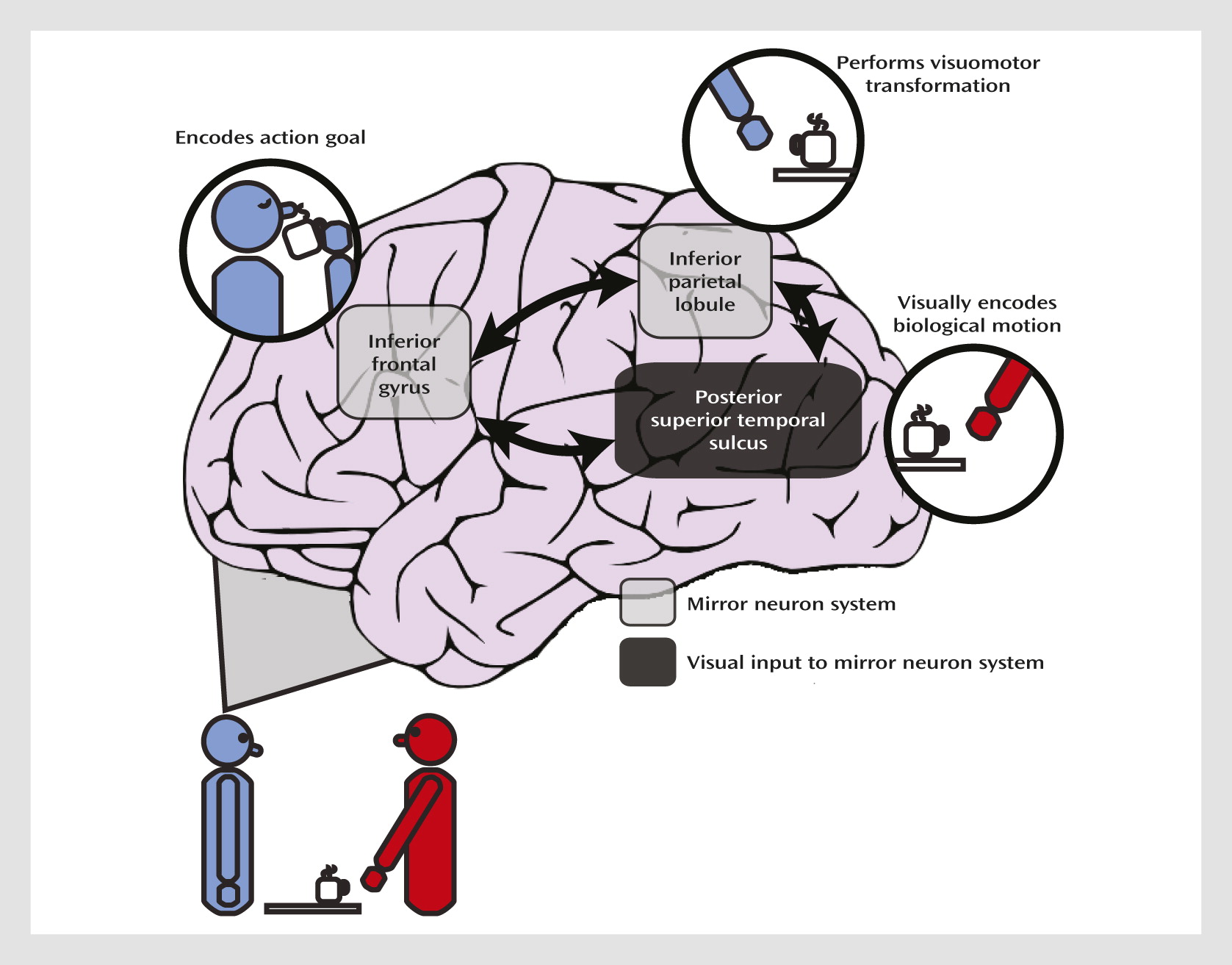
A further advantage to using imitation as a tool for studying social impairments is that neural correlates of action imitation are known at the cellular level. Neurophysiological studies of nonhuman primates have uncovered neurons, termed
mirror neurons, in the premotor and parietal cortices that increase their firing rate when the monkey executes or observes an action. This mirror neuron system is hypothesized to form the neural basis of mirror matching and action imitation (
Figure 1) (
7). Within this framework, parietal neurons code for motoric components of the action and inferior frontal regions code for the action goal. Functional MRI (fMRI) studies have revealed an analogous network of regions involved in action imitation in humans (
8). The posterior superior temporal sulcus, although it does not contain neurons with known mirror properties, is specialized for perceiving biological motion (
9), which refers to the unique visual phenomenon of movements produced by living things; the posterior superior temporal sulcus provides this higher-order visual input to the mirror neuron system. Both temporoparietal and frontal regions contribute to the “core circuit” for imitation (
7), and the mirror neuron system allows us to covertly simulate the sensation and movement of others.
To date, studies of imitation in schizophrenia have been sparse, but impaired imitation has been reported in facial emotional expressions (
14), as well as nonemotional facial and manual movements, which was found to be correlated with symptom severity (
15,
16). Recent evidence also suggests deficient automatic mimicry in schizophrenia (
17). Although no study has explicitly examined brain activity during action imitation in schizophrenia, abnormal posterior superior temporal activity during biological motion perception in schizophrenia has been reported (
18).
Our goal in this fMRI study was to examine activation in the mirror neuron system of individuals with schizophrenia and healthy comparison subjects. Given findings of impaired imitation ability in schizophrenia, we hypothesized that we would find abnormal activity in the inferior frontal gyrus, the inferior parietal lobule, and the region around the posterior superior temporal sulcus and that it would be related to clinical symptoms and social functioning.
Discussion
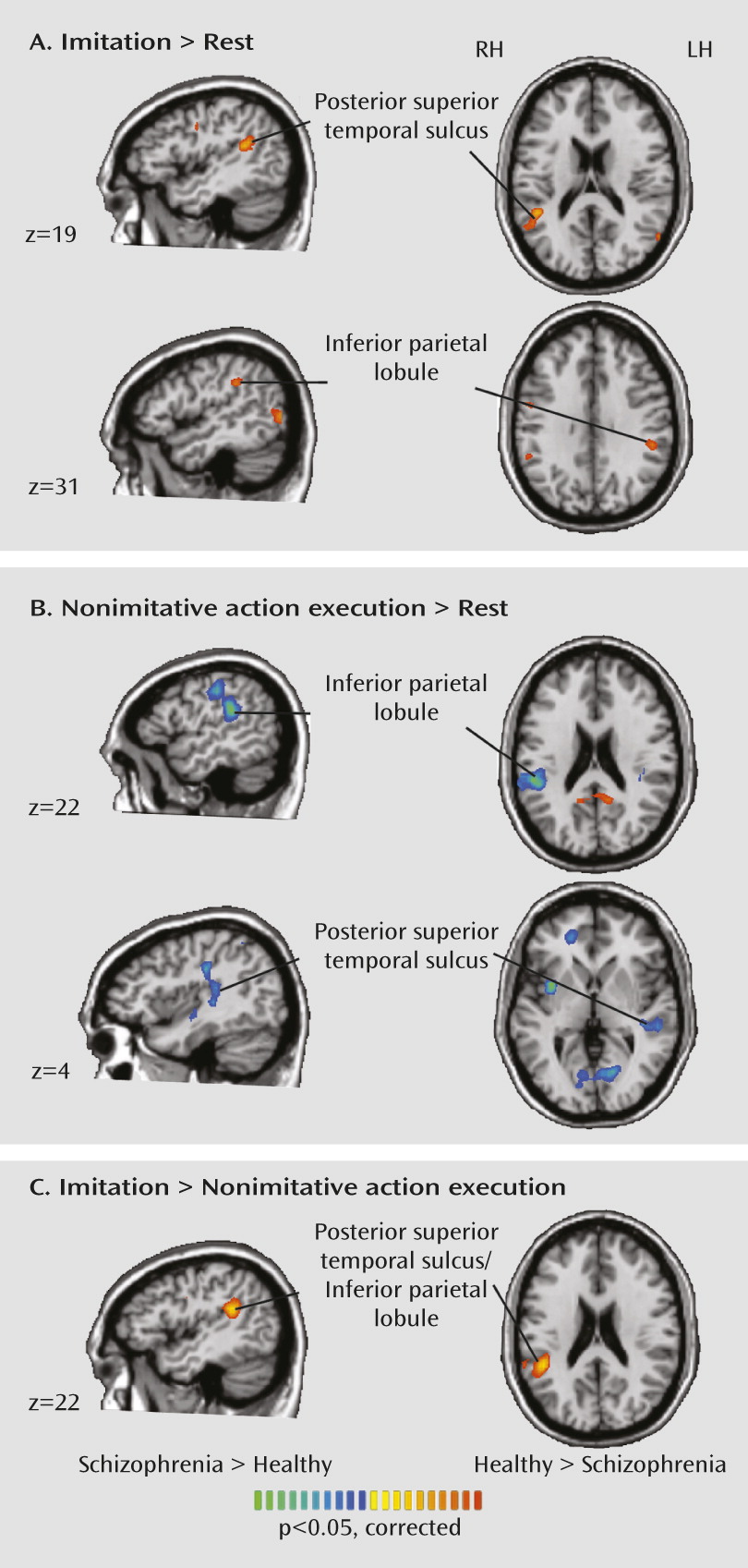
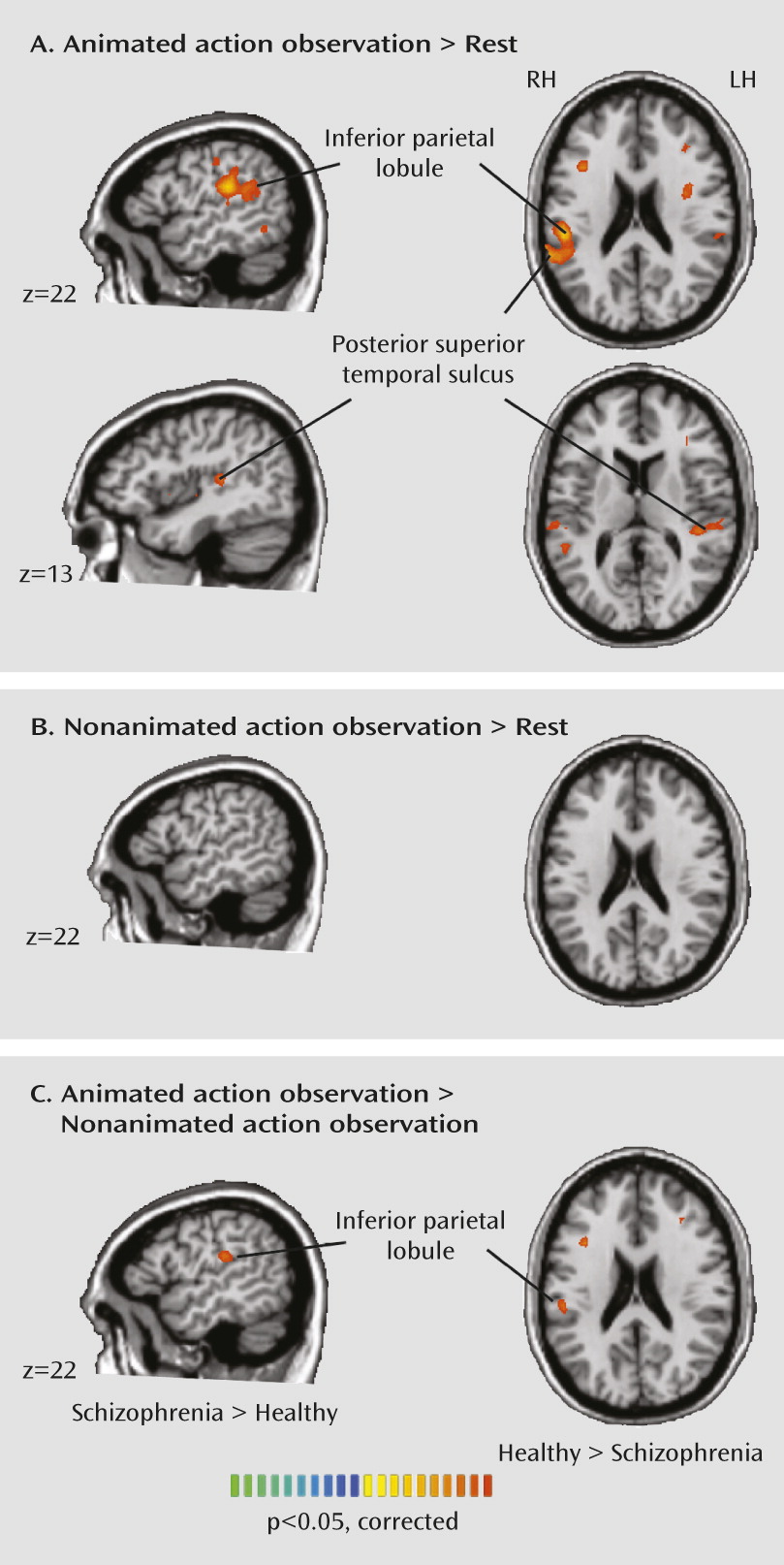
Our results indicate abnormal neural activity during action imitation and observation in patients with schizophrenia. Instead of a simple reduction in mirror neuron system activation in schizophrenia, we observed an interesting crossover effect. Patients showed a less differentiated response than healthy subjects in the inferior parietal lobe and posterior superior temporal sulcus during action imitation. Although patients had less activation than healthy subjects in the posterior superior temporal sulcus during imitation, they had greater activation in the posterior superior temporal sulcus and inferior parietal lobe during nonimitative action, suggesting that the mirror neuron system is less fine-tuned in schizophrenia. Patients also showed reduced activation in the inferior parietal and posterior superior temporal regions during observation of a moving hand.
In interpreting the significance of these findings, we turn to accounts of posterior superior temporal sulcus and inferior parietal lobe function in the healthy brain. The posterior superior temporal sulcus plays a crucial role in biological motion perception (
9,
26) and provides higher-order visual input necessary for imitation to the mirror neuron system. Accordingly, abnormal activation in this region during imitation and observation in schizophrenia suggests that impaired imitation (
14–
16) may have its basis largely in faulty perceptual input to brain regions more directly implicated in imitation. Our results bear a striking similarity to earlier findings (
18) showing abnormal posterior superior temporal sulcus activity during biological motion perception in schizophrenia. In that study, patients tended to perceive randomly moving dots as having biological attributes. Failure to discriminate biological from nonbiological motion in schizophrenia was reflected in the posterior superior temporal sulcus, with no differential activity in this region between biological and nonbiological motion perception.
We also observed abnormal activation in patients in the nearby inferior parietal lobe, which is densely interconnected to the posterior superior temporal sulcus (
27). Based on the involvement of the inferior parietal lobe in action imitation and observation (
7), apraxia resulting from inferior parietal lobe lesions (
28), and the multimodal nature of representations stored in the inferior parietal lobe (
29), a unique role for this region has been postulated. The inferior parietal lobe is argued to be involved in matching the perceptual information about an observed action with a motoric representation that action; this function gives rise to the ability to understand actions, and ultimately, intentions. This claim is bolstered by fMRI findings of involvement of the temporoparietal junction, comprising the inferior parietal lobe and superior temporal regions, during more complex theory-of-mind tasks (
30). We observed greater activity in the right inferior parietal lobe during nonimitative actions and reduced activity in the inferior parietal lobe during imitative actions and action observation in patients, which is suggestive of reduced fine-tuning of this region for socially relevant stimuli in schizophrenia.
Mounting evidence implicates inferior parietal dysfunction in schizophrenia. In a review, Torrey (
31) highlighted the late development of this region, both evolutionarily and over the lifespan, which is consistent with the late onset of the disease and the notion that schizophrenia is a uniquely human disorder (
32). Moreover, the inferior parietal lobe is crucial for self-related processes (e.g., perception of agency) central to illness phenomenology. Inferior parietal lobe
hyperactivity has been observed in multiple cognitive and sensorimotor tasks in schizophrenia (see reference
31 for a review). Furthermore, the most robust functional connectivity abnormalities in schizophrenia across the brain occur between parietal nodes, in which increased connectivity has been reported (
33). Our findings are consistent with abnormal parietal lobe function in schizophrenia.
Increasing evidence suggests impaired imitation in schizophrenia, but what implications do these findings have for understanding the clinical picture, and how do our findings of abnormal neural activity during action imitation elucidate the underlying pathology? One route to understanding the minds of others is via internalized mimicking. Additionally, automatic mimicry enhances the subjective quality of social interactions (
5). One might postulate that disturbances in imitation could cascade into a failure to understand the minds of others (
16) and that poor social interactions could fuel social isolation, which in turn can worsen symptoms (
34).
Given the purported role of the inferior parietal lobe and posterior superior temporal sulcus as key nodes in an imitation network, findings of abnormal activity in these regions in schizophrenia suggest that imitation impairments may have their basis in faulty biological motion perception and transformation of visual information into motor representations. Hyperactivity in these regions during nonimitative action execution and hypoactivation of these regions during imitation generates interesting hypotheses about the nature of social impairments in schizophrenia. The combination of hyper- and hypoactivation in these regions may correspond to the clinical presentation of social dysfunction in schizophrenia, which is characterized by both enhanced sensitivity to social information (e.g., suspiciousness about others, perceiving animacy and intentions in inanimate objects) and reduced sensitivity to the states of others (e.g., social withdrawal, avolition). We observed correlations that bordered on significance between symptom severity and neural activity in the posterior superior temporal sulcus and inferior parietal lobe during both imitative and nonimitative actions. It should be noted, however, that given the exploratory nature of these correlations, they were not corrected for multiple comparisons, and larger and more clinically heterogeneous samples are required to fully explore this link.
One important question for future study is the developmental origin of imitation impairments in schizophrenia. One possibility is that imitation ability fails to develop appropriately. Another is that mirror-matching abilities break down during illness onset. It is known that experience in the form of sensorimotor learning can mediate the automaticity of imitation (
35). If the link between motor commands and sensory consequences becomes degraded in schizophrenia, perhaps via alterations in corollary discharge (
36), one could envision a gradual breakdown in mirror-matching. Furthermore, dysfunctional simulation of the intentions of others would spur the need for alternative sources of information, perhaps through prior knowledge. Indeed, Chambon et al. (
37) found that schizophrenia patients were more likely to use prior expectations over current information when deciphering the actions of others. While this notion is speculative, it forms a basis for further inquiry.
Although inferior frontal activation did not significantly differ between healthy subjects and schizophrenia patients for any of the contrasts of interest, we observed a significant correlation between inferior frontal activation and schizotypal traits in healthy participants. Score on the interpersonal subscale of the Schizotypal Personality Questionnaire was associated with increased inferior frontal activity during imitation. These results suggest that healthy individuals with elevated schizotypy may be expending greater effort to correctly imitate the actions of others. Platek et al. (
38) also observed subtle imitative abnormalities in relation to elevated schizotypy.
There are several limitations to our study. First, the patients were medicated. Although the effects of antipsychotic medication on imitation and action observation are unknown, antipsychotic dosage was related to inferior parietal lobe activity in the imitation versus nonimitative action contrast. That is, brain activity in patients with higher medication dosages looked more like that of healthy subjects, suggesting a possible therapeutic effect of antipsychotic treatment. Second, there were subtle group differences in task performance; however, accuracy was high in both groups, response time differences were small, and there was no difference in the total number of movements made, suggesting that differences in brain activation cannot be explained by the amount of motor output. Finally, we did not independently measure attention during action observation. However, the brain activity during observe trials was markedly different from that during rest blocks, and groups were matched on IQ, which precludes the possibility of gross differences in cognitive functioning.