Cognitive Training in Mental Disorders: Update and Future Directions
Abstract
Objective
Method
Results
Conclusions
Introduction
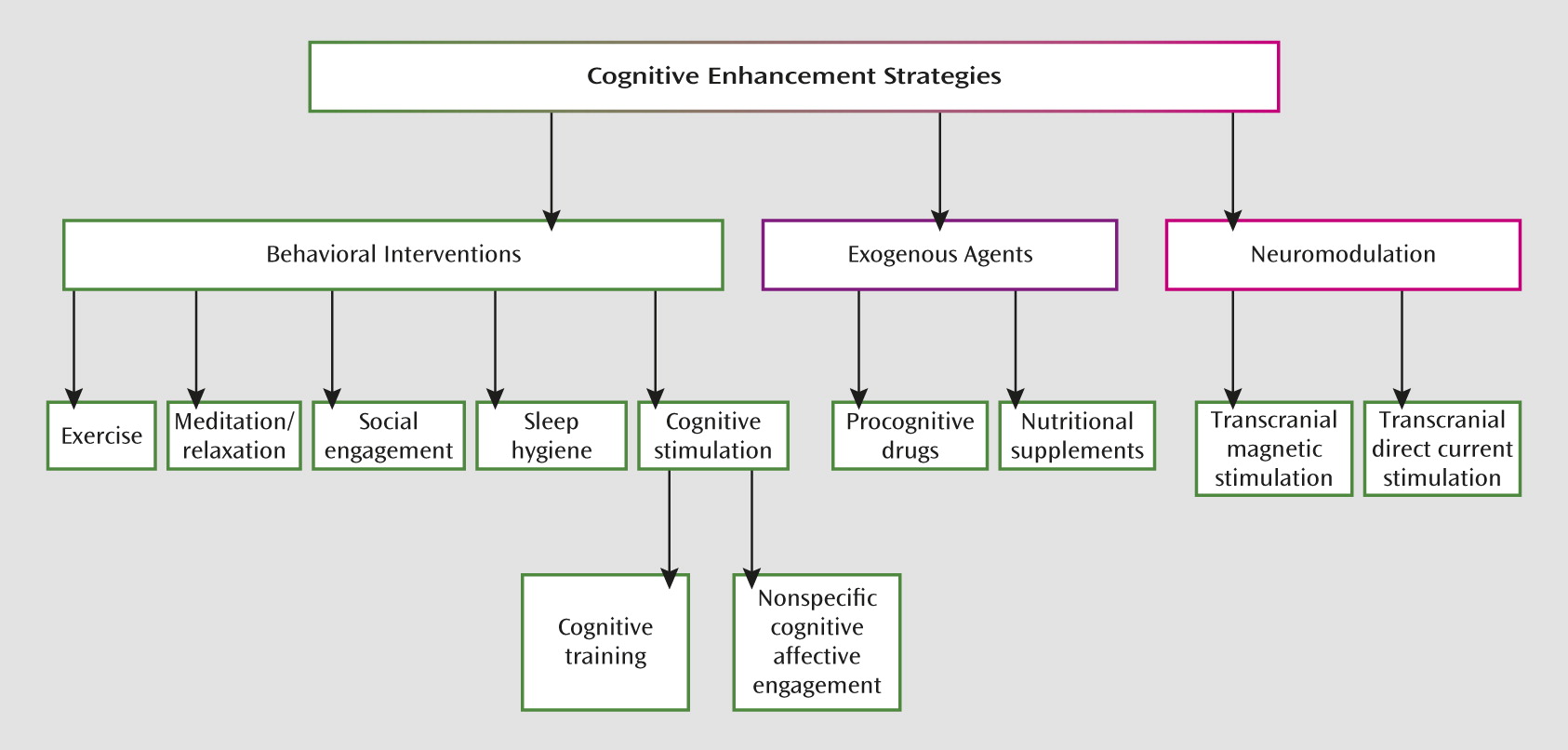
How is Cognitive Training Defined?
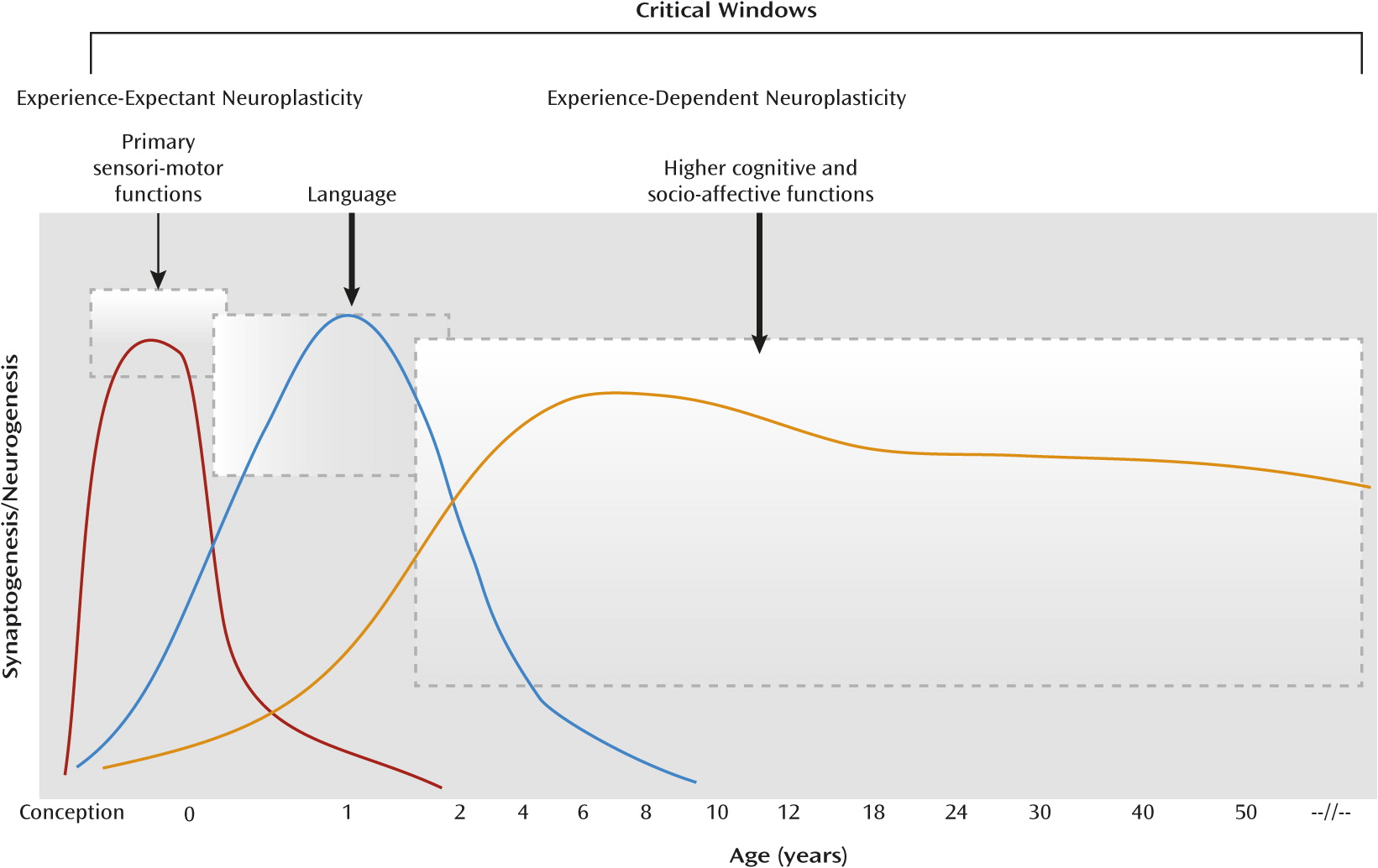
Mental Illness, Neuroplasticity, and Cognitive Training
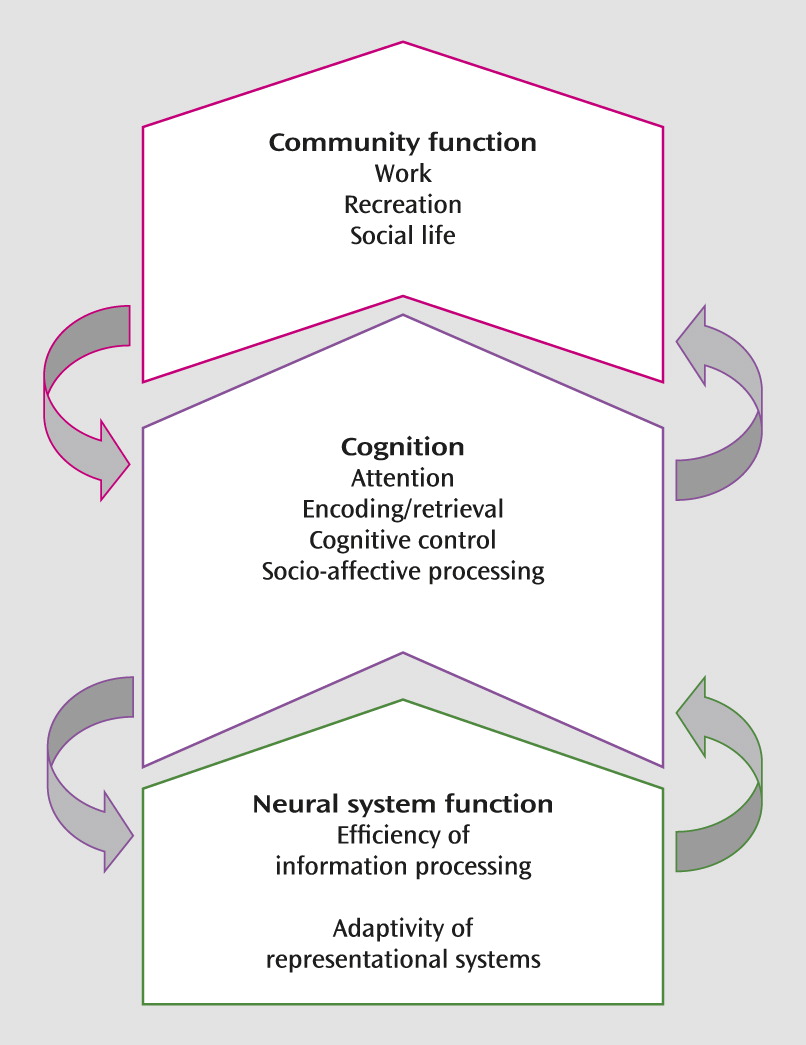
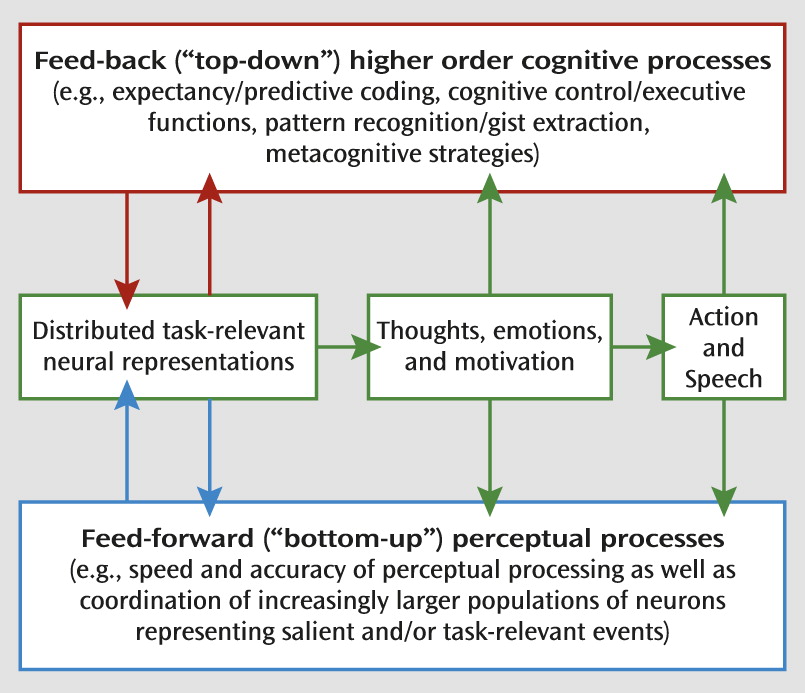
Neural Targets for Cognitive Training in Mental Illness
Current State of Knowledge
Schizophrenia.
Attention deficit hyperactivity disorder (ADHD).
Anxiety disorders.
Mood disorders.
Substance use disorders.
Autism spectrum disorders (ASDs).
Other disorders.
Predictors and Moderators of Response to Cognitive Training
Learner Characteristics
Age and neurodevelopmental effects.
Genotype.
Cognitive function and brain reserve.
Motivation and emotional state.
Features of Training
Training approaches.
Intensity and progression of training.
Adjunctive Cognitive-Enhancing Interventions
Physical exercise.
Pharmacological agents.
Brain neuromodulation.
Trial Design Issues
| Item | Description |
|---|---|
| Participant characterization | Are potential predictors/moderators (e.g., baseline cognitive function, psychopathology, and neural reserve) assessed? |
| Are inclusion/exclusion criteria (e.g., presence of targeted cognitive capacity/deficits) justified? | |
| Intervention targets | Are cognitive targets (deficits/capacities) linked to clinical status and functioning? |
| Do the cognitive training interventions match the perceptual/cognitive/affective processes that characterize the disorder and/or neural circuits implicated? | |
| Is the hypothesized therapeutic mechanism supported by research and theory? | |
| Outcome assessment | Are potential predictors/moderators (e.g., medications, therapist engagement) of outcomes considered? |
| Do assessments provide for the elucidation of intervention mechanisms (e.g., temporal precedence between putative mediators/mechanisms and target outcomes)? | |
| Are retention/completion rates assessed and reported? | |
| Are cognitive/functional outcomes distinguishable from practice effects? | |
| Are valid measures of proximal (e.g., performance on training tasks, neurocognitive measures) and more distal outcomes (clinical status, functioning, adverse effects, durability, generalization of cognitive and affective outcomes distinct from training tasks) included? | |
| Does the plan include measures at multiple levels of analysis (e.g., genes, molecules, cells, circuits, physiology, behavior, and self-report) as appropriate (64). | |
| Concomitant treatments | Is cognitive training intended as a monotherapy or as an adjunctive treatment? Are concomitant treatments considered in the assessment and analysis plan? |
| How might the proposed concomitant therapies potentiate (e.g., promoting plasticity; generalization of skills) or interfere with (e.g., medication side effects) cognitive training effects? | |
| Are concomitant treatments held constant across treatment conditions and/or quantified and considered in analyses? | |
| Comparison condition | Is the comparison condition justified in terms of the research question and stage of intervention development/testing? |
| Does the comparison condition control for attention, expectations, and potential practice effects associated with training/assessment protocols, as appropriate? | |
| Scalability/potential for dissemination | Are all relevant stakeholders considered (i.e., patients/families [e.g., acceptability], clinicians [availability of an appropriately trained workforce], and policymakers [competing demands, therapist time/involvement, and other costs])? |
| What are the implementation strategies (e.g., delivery within existing services, such as employment training; use of Internet or other facilitative technology for conducting assessments and delivering the intervention; provisions to facilitate motivation/engagement)? | |
| Design considerations | Are randomization procedures clearly detailed and justified? |
| Are intervention protocols standardized and manualized? | |
| Are there plans to monitor fidelity and operationalize the delivery of the experimental and comparison conditions? | |
| Are statistical approaches state of the art and appropriately matched to the research question and data structure? |
Conclusions and Future Directions
Footnote
References
Information & Authors
Information
Published In
History
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing PsychiatryOnline@psych.org or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

