Treatment-Resistant Bipolar Depression: A Randomized Controlled Trial of Electroconvulsive Therapy Versus Algorithm-Based Pharmacological Treatment
Abstract
Objective:
Method:
Results:
Conclusion:
Method
Overview
Subjects
Treatments
Electroconvulsive therapy.
Algorithm-based pharmacological treatment.
Randomization and Masking of Study Groups
Assessments
Outcome Measures
Ethics
Statistics
Descriptive analyses.
Missing data.
Efficacy analyses.
Results
Patient Flow and Characteristics
| Measure | ECT (N=38) | Algorithm-Based Pharmacological Treatment (N=35) | Analysis | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | df | p | |
| Age at study inclusion (years) | 48.0 | 10.1 | 48.4 | 13.2 | 0.16 | 71 | 0.88 |
| Age at illness onset (years) | 15.9 | 6.7 | 19.0 | 11.3 | 1.34 | 47.3 | 0.19 |
| Duration of illness (years) | 31.8 | 12.2 | 27.7 | 10.6 | –1.45 | 65 | 0.16 |
| Number of episodes | |||||||
| Depressive | 22.3 | 24.2 | 17.4 | 14.1 | –0.94 | 57 | 0.36 |
| Hypomanic | 18.2 | 30.8 | 9.6 | 8.1 | –1.51 | 36.2 | 0.14 |
| Manic | 2.7 | 8.6 | 1.1 | 2.6 | –0.94 | 58 | 0.36 |
| Psychotic | 1.6 | 2.7 | 3.3 | 9.7 | 1.00 | 60 | 0.33 |
| Scores on rating scales | |||||||
| Montgomery-Åsberg Depression Rating Scalea | 39.1 | 7.5 | 38.0 | 7.4 | –0.60 | 71 | 0.55 |
| 30-item Inventory of Depressive Symptomatology–Clinician-Ratedb | 48.9 | 9.7 | 46.5 | 13.6 | –0.83 | 64 | 0.41 |
| Young Mania Rating Scalec | 3.5 | 2.8 | 3.2 | 2.3 | –0.55 | 71 | 0.59 |
| Clinical Global Impression for Bipolar Disorderd | 5.8 | 0.7 | 5.8 | 0.7 | –0.48 | 68 | 0.64 |
| N | % | N | % | χ2 | df | p | |
| Male gender | 21 | 55.3 | 16 | 45.7 | 0.67 | 1 | 0.42 |
| Bipolar disorder type I | 14 | 36.8 | 15 | 42.9 | 0.28 | 1 | 0.60 |
| Lifetime medication use | |||||||
| Antipsychotics | 30 | 78.9 | 30 | 87.5 | 0.57 | 1 | 0.55 |
| Antidepressants | 35 | 92.1 | 33 | 94.3 | 0.14 | 1 | 1.00 |
| Anticonvulsants | 33 | 86.8 | 25 | 71.4 | 2.65 | 1 | 0.15 |
| Lithium | 12 | 31.6 | 20 | 51.7 | 4.84 | 1 | 0.04 |
Treatment Variables
| Patient | Lithium: Mean Dosage (mg/day)a | Anticonvulsants, With Mean Dosage (mg/day)b | Antipsychotics, With Mean Dosage (mg/day) | Antidepressants (Classc), With Mean Daily Dosage (mg/day) | Time of Study Exit (week) | MADRSd Score at Study Exit |
|---|---|---|---|---|---|---|
| 1 | 124.5 | Lamotrigine, 300.0 | Venlafaxine (SNRI), 300.0 | 5 | 15 | |
| 2 | 166.0 | Lamotrigine, 37.5 | Quetiapine, 300.0 | Sertraline (SSRI), 75.0 | 6 | 5 |
| 3 | 210.0 | Valproate, 1200.0 | Olanzapine, 5.0 | Citalopram (SSRI), 20.0 | 5 | 21 |
| 4 | 193.0 | Lamotrigine, 58.3 | Quetiapine, 120.0 | 5 | 18 | |
| 5 | 207.5 | Quetiapine, 566.7 | Escitalopram (SSRI), 70.0 | 6 | 24 | |
| 6 | 45.5 | Lamotrigine, 41.7 | Perphenazine, 8.0 | Venlafaxine (SNRI), 225.0 | 6 | 24 |
| 7 | 156.8 | Lamotrigine, 800.0 | Olanzapine, 2.5 | Bupropion (NDRI), 150.0 | 5 | 20 |
| 8 | 166.0 | Lamotrigine, 100.0 | Olanzapine, 7.5 | Fluoxetine (SSRI), 20.0 | 6 | 3 |
| 9 | 332.0 | Lamotrigine, 45.0 | Quetiapine, 180.0 | Mirtazapine (NaSSa), 60.0 | 6 | 35 |
| 10 | 53.7 | Lamotrigine, 62.5 | Citalopram (SSRI), 12.0; venlafaxine (SNRI), 181.3 | 6 | 29 | |
| 11 | 166.0 | Olanzapine, 5.0 | Fluoxetine (SSRI), 32.0; paroxetine (SSRI), 10.0e | 6 | 30 | |
| 12 | Lamotrigine, 316.7 | Quetiapine, 283.3 | 6 | 22 | ||
| 13 | Lamotrigine, 200.0; valproate, 1500.0 | Olanzapine, 5.0 | Fluoxetine (SSRI), 40.0 | 6 | 15 | |
| 14 | Lamotrigine, 83.3 | Quetiapine, 204.2 | Mirtazapine (NaSSa), 27.5 | 6 | 10 | |
| 15 | Lamotrigine, 41.7 | Quetiapine, 75.0 | 6 | 11 | ||
| 16 | Lamotrigine, 25.0 | Quetiapine, 250.0 | 0 | 44 | ||
| 17 | Lamotrigine, 466.7 | Quetiapine, 208.3 | 5 | 4 | ||
| 18 | Lamotrigine, 350.0 | Olanzapine, 30.0 | Fluoxetine (SSRI), 40.0 | 0 | 57 | |
| 19 | Lamotrigine, 75.0; valproate, 900.0 | Olanzapine, 10.0 | Mirtazapine (NaSSa), 15.0 | 5 | 3 | |
| 20 | Lamotrigine, 16.7; valproate, 850.0 | Quetiapine, 383.3 | Mirtazapine (NaSSa), 30.0 | 6 | 21 | |
| 21 | Lamotrigine, 300.0 | Olanzapine, 5.0 | Escitalopram (SSRI), 20.0; mirtazapine (NaSSa), 15.0 | 6 | 43 | |
| 22 | Lamotrigine, 68.8 | Quetiapine, 300.0 | 5 | 25 | ||
| 23 | Lamotrigine, 100.0 | Quetiapine, 133.3 | 6 | 9 | ||
| 24 | Lamotrigine, 300.0 | Quetiapine, 100 | Venlafaxine (SNRI), 120.0; bupropion (NDRI), 150.0 | 6 | 23 | |
| 25 | Lamotrigine, 10.0; valproate, 1500.0 | Quetiapine, 50.0 | Bupropion (NDRI), 150.0 | 1 | 29 | |
| 26 | Lamotrigine, 10.0; valproate, 600.0 | Quetiapine, 110.0; olanzapine, 10.0 | 6 | 8 | ||
| 27 | Lamotrigine, 40.0 | Olanzapine, 20.0 | Fluoxetine (SSRI), 28.0 | 5 | 28 | |
| 28 | Lamotrigine, 41.7 | Quetiapine, 158.3 | Bupropion (NDRI), 205.0 | 3 | 51 | |
| 29 | Lamotrigine, 12.5; valproate, 600.0 | Quetiapine, 25.0 | 0 | 20 | ||
| 30 | Lamotrigine, 30.0; valproate, 450.0 | Olanzapine, 5.0; quetiapine, 450.0 | Venlafaxine (SNRI), 37.5f | 6 | 21 | |
| 31 | Lamotrigine, 115.0 | Olanzapine, 12.0 | 6 | 19 | ||
| 32 | Lamotrigine, 120.8; valproate, 1300.0 | Aripiprazole, 15.0g | 6 | 25 | ||
| 33 | Quetiapine, 545.8 | 6 | 21 |
Efficacy
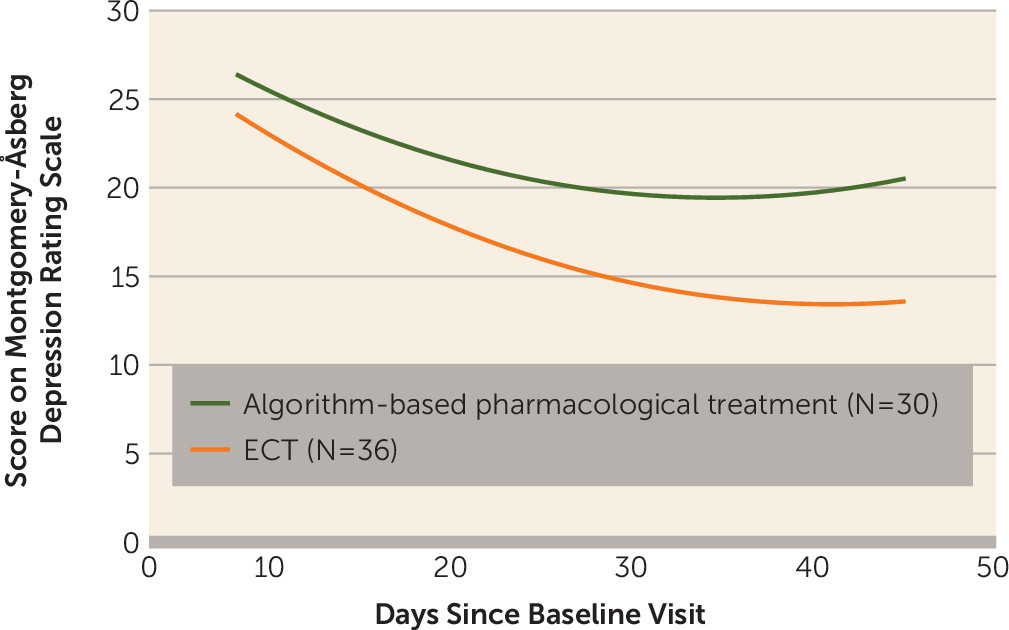
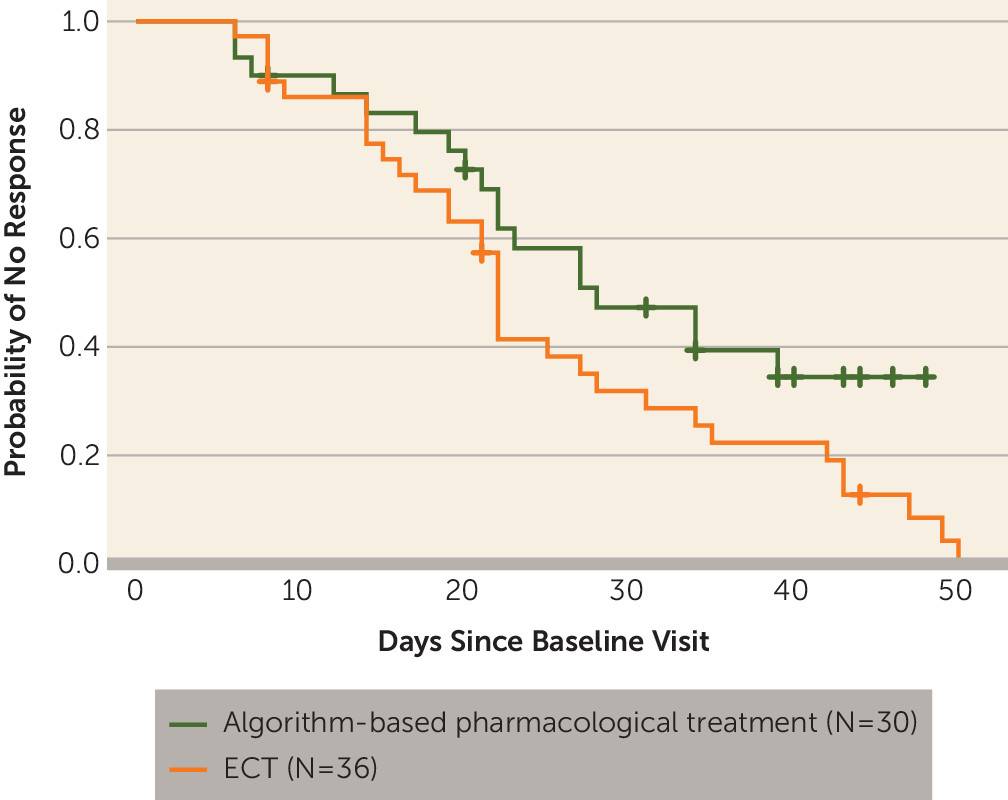
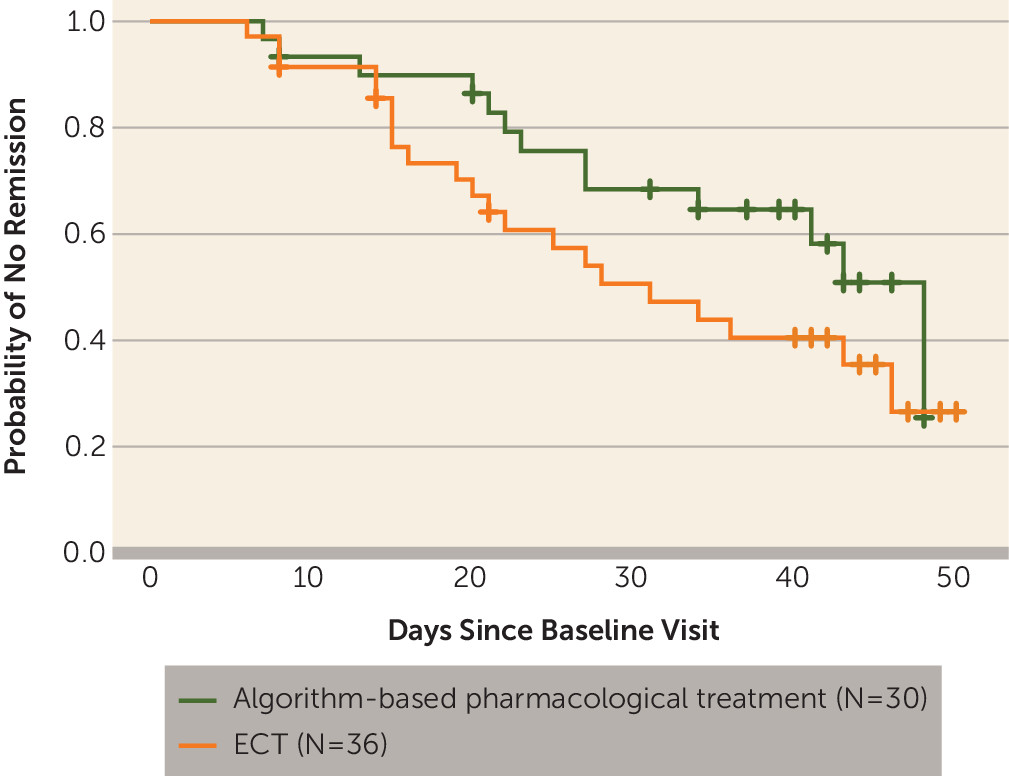
Response and Remission Rates
Adverse Events
| Symptom | ECT (N=36) | Algorithm-Based Pharmacological Treatment (N=30) | Relation to Treatment Procedure |
|---|---|---|---|
| Psychic adverse events | |||
| Concentration difficulties | 3 | Improbable | |
| Asthenia, lassitude, or increased fatigability | 1 | 2 | Improbable |
| Sleepiness or sedation | 3 | Possible | |
| Failing memory | 2 | 2 | Probable for the patients in the ECT group |
| Depression | 1 | Improbable | |
| Tension or inner unrest | 1 | 1 | Possible for the patient in the pharmacological group, probable for the patient in the ECT group |
| Increased duration of sleep | 1 | Possible | |
| Reduced duration of sleep | 2 | Possible | |
| Increased dream activity | 1 | Possible | |
| Emotional indifference | 1 | Possible | |
| Neurologic adverse event | |||
| Epileptic seizures | 1 | Improbable, patient had a complex partial seizure disorder from childhood that was unknown to the treating clinicians at inclusion in the study | |
| Autonomic adverse events | |||
| Reduced salivation | 1 | Possible | |
| Nausea or vomiting | 1 | Possible | |
| Constipation | 1 | Probable | |
| Orthostatic dizziness | 2 | Probable | |
| Increased tendency to sweat | 3 | 1 | Possible for the patient in the pharmacological group, improbable for the patients in the ECT group |
| Other adverse events | |||
| Rash | 1 | Probable | |
| Weight gain | 1 | Possible | |
| Weight loss | 1 | Improbable | |
| Diminished sexual desire | 2 | 4 | Possible |
| Orgasmic dysfunction | 1 | Possible | |
| Headache | 1 | Probable | |
| Death (due to an overdose of illicit drugs after discharge from hospital) | 1 | Improbable | |
| Medication overdose with possible suicidal ideation | 1 | 2 | Improbable |
| Self-strangulation attempt | 1 | Improbable | |
| Possible suicide attempt (patient jumped from a cliff) | 1 | Improbable | |
| Tooth damage | 1 | Probable | |
| Rib fracture | 1 | Improbable; the rib fracture occurred as the result of an accident unrelated to treatment |
Discussion
Footnotes
References
Information & Authors
Information
Published In
History
Authors
Author Contributions
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

