Response inhibition is assumed to be a key deficit underlying attention deficit hyperactivity disorder (ADHD) (
1). However, a meta-analysis showed only medium effect sizes (g=0.62) for response inhibition deficits in ADHD (
2), with large interindividual differences. Indeed, around half of individuals with ADHD have a response inhibition performance overlapping that of healthy comparison subjects (
2). Similar behavioral task outcomes can be due to different neural mechanisms. For example, neural correlates of reversal learning performance differed between participants with severe mood dysregulation and those with pediatric bipolar disorder despite similar task performance (
3). We therefore postulated that neural measures may be a more robust method than task performance to investigate the nature of response inhibition alterations in individuals with ADHD (
4).
Neuroimaging research in healthy subjects has identified a core network of brain regions involved in response inhibition, including a frontal-striatal network (the inferior frontal gyrus, the presupplementary motor area, basal ganglia, and suprathalamic nucleus [
5,
6]) and a frontal-parietal network (the inferior frontal, superior frontal, and temporal/parietal areas [
7–
9]). The inferior frontal gyrus, generally linked to salient cue detection (
10), is thought to initiate the inhibition process, which is further executed by the presupplementary motor area and basal ganglia (
11–
13). Temporal/parietal and superior frontal nodes are thought to underlie the top-down direction of attentional resources during response inhibition (
7,
14). Additionally, anterior cingulate areas are involved in error processing, as indicated by activation following failed response inhibition (
15). While attention and error processing are not specific to response inhibition (
16), deficits in these processes influence response inhibition performance (see, for instance, reference
17).
Given these inconsistent previous findings, as well as relatively small study groups in earlier studies (
4,
26), the first aim of our study was to investigate neural activation patterns underlying response inhibition in a large group of adolescents and young adults with and without ADHD. Reaction time on the stop-signal task was used as a behavioral index of response inhibition performance, reaction time variability as a measure of attention (
16), and error rate as a measure of error processing (
15). We expected hypoactivation in the frontal-striatal and frontal-parietal networks during both successful and failed response inhibition in individuals with ADHD (
23,
30) and expected the degree of hypoactivation to be associated with ADHD severity. Inhibition-related activation in frontal areas was expected to correlate with stop-signal reaction times (
5), activation of parietal nodes with reaction time variability (
7), and error rates with anterior cingulate activation after failed inhibition (
31).
As a second aim, we investigated the suitability of response inhibition as an ADHD endophenotype by comparing neural correlates of response inhibition in adolescents with ADHD, their unaffected siblings, and healthy comparison subjects. Endophenotypes are heritable markers more closely related to the genetic underpinnings of a disorder than the disorder itself (
32), which may facilitate the search for causal genetic variants of a disorder (
33). Assuming neural activation to be causally closer to the genetic factors underlying ADHD than task-outcome measures, we expected unaffected siblings to demonstrate neural hypoactivation in frontal-striatal nodes that was intermediate between the activation of the probands and the comparison subjects, even in the absence of behavioral deficits (
34). Unaffected siblings may further be able to recruit alternative neural mechanisms to compensate for impaired response inhibition, which would show as hyperactivation outside of the response inhibition network, specifically in parietal areas (
35–
37).
Results
Task Outcome Measures
A main effect of diagnostic group on stop-signal reaction time (see
Table 1) was found, indicating longer reaction times in participants with ADHD than in unaffected siblings (β=−15.4, p=0.015) and in comparison subjects (β=−13.8, p=0.05). Probands made significantly more errors on the go trials than unaffected siblings (β=−1.8, p<0.013) and comparison subjects (β=−2.5, p<0.001). Stop-signal reaction time and error rate did not differ between the latter two groups. Regarding reaction time variability, probands performed worse than their unaffected siblings (β=−15.6, p<0.001), who performed worse than the comparison subjects (β=−9.5, p<0.021). No influences of age, gender, IQ, medication status, or comorbid diagnoses were found.
fMRI Task Activation
Activation maps for the successful stop-go, failed stop-go, and failed stop–successful stop contrasts across all participants are shown in Figure S1 in the online data supplement. In the successful stop condition we observed higher beta values in the bilateral inferior frontal cortex, as well as in the bilateral insula, right frontal pole and middle frontal gyrus, right thalamus and caudate nucleus, bilateral anterior and posterior cingulate, medial frontal gyrus, bilateral temporal/parietal junction and lateral occipital areas, left hippocampus, and cerebellum. Similar patterns were observed in the failed stop condition, with higher beta values in the left inferior frontal gyrus, insula, and caudate nucleus merged into one cluster. The failed stop condition showed additional activation in the left frontal pole and superior frontal area.
The failed–successful stop condition showed increased betas in the bilateral calcerine occipital cortex, anterior cingulate, presupplementary motor area, and left inferior frontal gyrus (see Table S1 in the online data supplement). Activation maps for the diagnostic groups as well as difference maps for the go condition are also reported in the online data supplement; although the comparison subjects showed higher activation in the medial frontal pole during go trials, this activation did not overlap with group contrasts of interest nor did it survive multiple-comparison corrections.
Group Differences in fMRI Task Activation
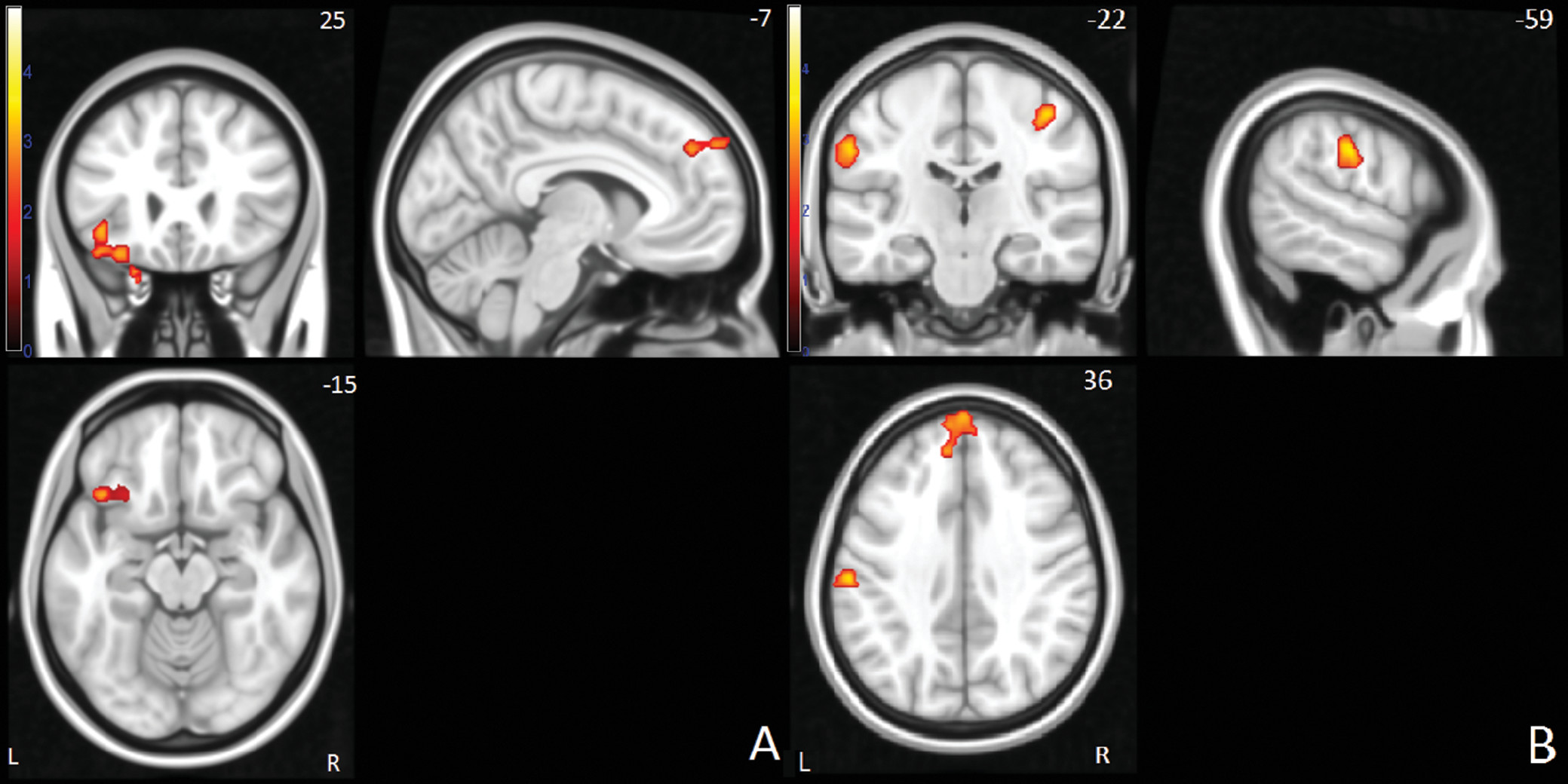
Between-group differences in neural activation for the successful stop-go condition were located in the left inferior frontal, superior frontal and anterior cingulate gyrus, left supramarginal gyrus, right postcentral gyrus, and right temporal/parietal junction (
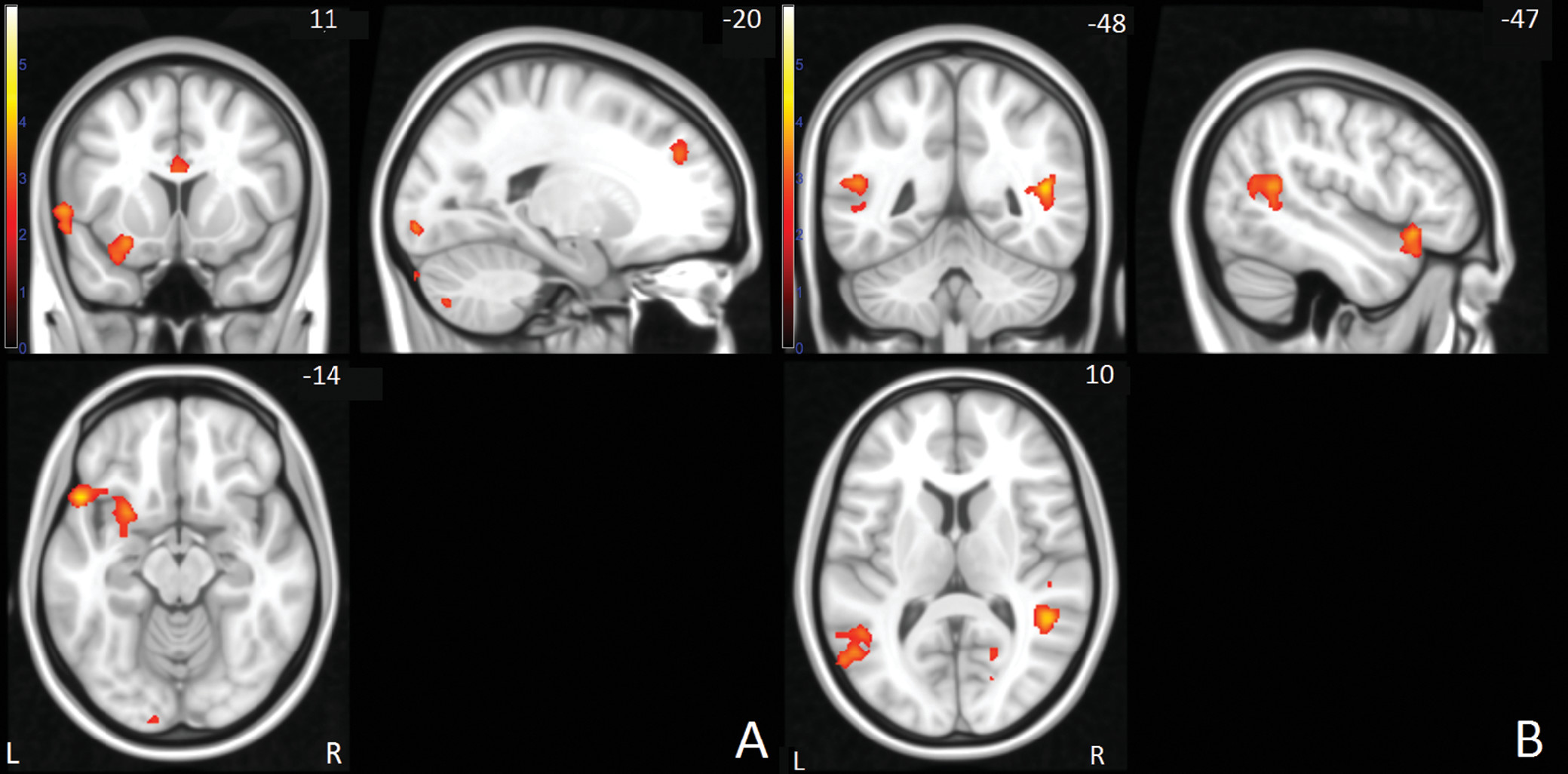
Figure 1). For the failed stop-go condition, between-group comparisons showed differences in the left inferior and superior frontal, anterior cingulate, left supramarginal, and bilateral temporal/parietal areas, as well as left cerebellum and right occipital areas (
Figure 2). An overview of all overall group effects and differences between the three diagnostic groups is shown in
Table 2.
For the successful stop condition, the siblings and probands showed less activation than the healthy comparison subjects in right temporal/parietal, left supramarginal, and right postcentral/supramarginal areas. In the left superior frontal and inferior frontal gyri, the probands showed less activation than both the siblings and comparison subjects, while the latter two did not differ (see also Figure S2 in the online data supplement).
In the failed stop condition, we observed levels of activation for siblings that were in between the levels observed for the probands and comparison subjects in bilateral temporal/parietal areas and the inferior frontal gyrus. In the anterior cingulate and left superior frontal gyri, probands and siblings showed similar levels of hypoactivation relative to the healthy comparison subjects. In the left supramarginal region, the siblings did not differ from the comparison subjects and showed higher activation than the probands (see also Figure S3 in the online data supplement).
Additionally, results from post hoc analyses relating neural activation to ADHD severity in the probands (
Table 3) indicated significant negative correlations between ADHD symptom count and neural activation in the inferior frontal gyrus during both successful and failed stop conditions, as well as in the superior frontal and temporal/parietal gyrus during the failed stop condition.
The failed stop–successful stop contrast did not reveal any significant differences between diagnostic groups.
Associations of Stop-Signal Task Outcomes With fMRI Task Activation
Shorter reaction times on the stop-signal task were significantly associated with higher levels of activation in the left inferior frontal and left superior frontal gyrus during successful stops (Table S2 in the online data supplement). Reaction time variability and error rate were not associated with activation in any of the nodes. Post hoc analyses confirmed the significant relation between inferior frontal activation and reaction time when examined in probands with ADHD and healthy comparison subjects separately, while the relation between stop-signal reaction time and superior frontal activation held only in the comparison subjects (see online data supplement). We further investigated the distribution of task performance and neural activation across the three groups. To this end, we compared the percentage of participants with ADHD and siblings who scored above the 90th percentile of the scores for the comparison group (see online data supplement). This analysis showed that 12% of the ADHD probands and 7% of the siblings showed task outcome (stop-signal reaction time) above the 90th percentile of the comparison group, while the elevated neural activation values across the different nodes showed on average 22% of probands’ and 19% of siblings’ values were above the 90th percentile of the comparison group (see Table S5 in the online data supplement for the comparison of individual nodes). This indicates that the within-group distributions of scores of the probands and siblings differed from that of the healthy comparison subjects more strongly for the neural activation than for the behavioral task outcome measures.
Covariate Effects and Sensitivity Analyses
No significant effects of age, scanner site, and gender were found in any of the neural nodes, nor were there interactions between diagnostic group and these covariates. In the online data supplement we present the outcomes of additional sensitivity analyses, in which we reexamined the main contrasts of interest while strictly correcting for IQ, gender, scan site, medication status, familial relations, comorbid disorders, stop-signal reaction time performance, and Conners’ scale scores. None of these factors significantly affected the reported main group differences in neural activation.
Discussion
This study provides several new insights into the relation between response inhibition performance, related neural activation, and ADHD. First, we demonstrated slower stop-signal reaction times and higher error rates along with hypoactivation in both the frontal-striatal and frontal-parietal networks in adolescents and young adults with ADHD. Second, we showed that the level of hypoactivation in these networks correlated with both stop-signal reaction time and ADHD severity. Third, we provided novel evidence for similar hypoactivation patterns in unaffected siblings, in the absence of behavioral response inhibition deficits (
19). Last, we showed that reaction time variability was higher in both probands with ADHD and unaffected siblings, suggesting a specific response inhibition deficit in ADHD and a broader cognitive impairment in both probands and siblings.
Over all groups, neural activation patterns during both successful and failed stop trials formed a network including the bilateral inferior frontal and superior frontal gyri, basal ganglia, and supramarginal areas. Activation in the inferior and superior frontal gyrus and basal ganglia nodes is in line with the response inhibition model proposed by Aron (
5). On the other hand, activation in temporal and parietal nodes, areas previously linked to attentional redirection and task-set maintenance (
45), likely reflects recruitment of attentional processes during response inhibition, in line with the models of Chambers et al. (
7) and Simmonds et al. (
9). The failed–successful stop contrast further revealed differential activation in visual areas, the anterior cingulate, and the inferior frontal cortex, consistent with previous findings, including a possible error processing component in anterior cingulate activation (
45). Inferior frontal activation in this contrast may reflect recruitment of additional resources to the response inhibition network (
5) or reflect more general cue updating after failed responses (
10). These results indicate that response inhibition is realized by activation in a large number of nodes from both frontal-striatal and frontal-parietal networks.
Adolescents and young adults with ADHD and their unaffected siblings showed levels of hypoactivation during both successful and failed stop trials, with unaffected siblings generally showing levels of activation similar to those of the probands or intermediate between those of the comparison subjects and probands with ADHD. Hypoactivation patterns were distributed across left superior, inferior, and medial frontal as well as bilateral temporal/parietal nodes, indicating a general and familial neural dysfunction across a large number of nodes attributed to distinct neural networks. Our results thereby confirm many preliminary previous findings in smaller groups of children and adolescents (
19–
24,
46,
47), which taken together describe very similar hypoactivation patterns in frontal-striatal and frontal-parietal areas (
26,
48). However, previous response inhibition studies in adults with ADHD demonstrated both neural hypo- and hyperactivation in the response inhibition networks (
21,
22,
26,
48). Our results indicate no evidence for hyperactivation, nor any interaction between neural activation and age. This suggests that at least in young adults the neural alterations are qualitatively similar to those in adolescents with ADHD and resemble alterations reported in children.
Activation in inferior and superior frontal as well as temporal/parietal areas was associated with ADHD severity, suggesting that multiple neural mechanisms are affected in both ADHD probands, as previously proposed in a meta-analysis (
26), and unaffected siblings. The hypoactivation in the left inferior and superior frontal gyrus were the only neural measures that correlated significantly with stop-signal reaction time length. This again fits relatively well with the model proposed by Aron (
5), who indicated the inferior frontal area as the central node in the response inhibition process. Both models by Aron (
5) and Chambers et al. (
7) have additionally indicated the dorsolateral prefrontal cortex as critical for top-down executive control, which would be the most likely explanation for the superior frontal hypoactivation in probands with ADHD in this area. However, since there was no condition manipulating attention or top-down control, these speculations cannot be directly derived from our data.
Hypoactivation in supramarginal and temporal/parietal regions of the probands with ADHD is also consistent with the models of Chambers et al. (
7) and Simmonds et al. (
9), which implicate these regions in attentional processes, which may influence the response inhibition process indirectly. These models are supported by evidence from transcranial magnetic stimulation studies showing attenuated attentional processing after parietal cortex stimulation (
49). However, none of the task outcome measures were related to neural activation in the parietal nodes. Therefore, more research targeting the temporal/parietal areas is necessary to establish their causal role in response inhibition and ADHD. Lastly, hypoactivation in anterior cingulate areas during the failed inhibition trials in both probands with ADHD and siblings suggests an additional deficit in response to perceived errors (
50). This appears consistent with the higher error rates found in probands with ADHD, although we found no direct association between error rates and anterior cingulate activation.
No group differences were found in the failed–successful stop contrast, indicating that there are no qualitative differences underlying inhibition failure between the diagnostic groups. Rather, the neurobiological nature of response inhibition deficits in ADHD is related to distributed hypoactivation in both frontal-striatal and frontal-parietal nodes during both task conditions.
The neural hypoactivation observed in unaffected siblings is largely in line with previous work (
19) that showed similar patterns of hypoactivation in inferior frontal and parietal areas in the absence of behavioral deficits in unaffected siblings of probands with ADHD, though this previous work reported atypical activation in the right instead of left inferior frontal gyrus for siblings. The intermediate activation levels in inferior frontal and temporal/parietal nodes specifically fit with previous work addressing the heritable nature of inferior frontal activation (
51). However, the predicted pattern of intermediate activation in siblings was present only during the failed stop condition, and it was not found in the superior frontal and supramarginal regions. Thus, although no direct evidence was found for differential activation between the successful and failed conditions, activation during the failed stop trials nonetheless appeared most sensitive in distinguishing probands with ADHD, unaffected siblings, and healthy comparison subjects.
The absence of behavioral response inhibition deficits in unaffected siblings is in contrast with previous behavioral literature (
52), including evidence from our own sample at an earlier time (
53). This current finding suggests that developmental factors are important in the investigation of familial patterns of response inhibition deficits, as unaffected siblings possibly show improved response inhibition performance during adolescence, while probands with ADHD do not (
54).
Of note is that the effect sizes of behavioral versus neural measures suggest that the differences in neural activation during response inhibition between diagnostic groups are more robust than stop-signal reaction time differences. Comparisons of the distributions of behavioral and neural outcome measures likewise indicate that neural hypoactivation is also more consistently present than response inhibition deficits in probands with ADHD. The strong differences in reaction time variability between probands with ADHD, their unaffected siblings, and healthy comparison subjects have effect sizes comparable to those obtained from the neural measurements. These results confirm earlier studies regarding reaction time variability as an endophenotype for ADHD (
55–
57). An alternative interpretation of the neural hypoactivation found in probands with ADHD could be that the deficits in reaction time variability in both probands and their siblings are related to a general attentional deficit, mediated by temporal/parietal hypoactivation. However, there was no significant correlation between neural activation in parietal areas and reaction time variability, which makes this interpretation less likely.
No direct evidence for compensatory neural activation in either subjects with ADHD or unaffected siblings was found. The finding of hypoactivation in unaffected siblings in the absence of behavioral response inhibition deficits or compensatory neural mechanisms warrants further attention. Additional research is needed to establish whether the siblings recruit compensatory resources that could not be detected with the current paradigm or whether the hypoactivation observed in siblings is unrelated to the response inhibition process or insufficient to cause behavioral deficits. Specifically, functional connectivity measures may offer additional insight into the possible recruitment of alternative neural resources in unaffected siblings.
To summarize the group differences, hypoactivation in inferior frontal, superior frontal, and temporal/parietal regions all independently explained variance in ADHD severity, with unaffected siblings showing intermediate patterns of hypoactivation in these areas during failed but not successful inhibition and in the absence of behavioral deficits. These findings support the familial nature of the response inhibition process in ADHD and suggest that the neural activation measures in these regions could be useful as possible endophenotypes for ADHD, although only the failed inhibition contrast showed a clear distinction between all three diagnostic groups.
It should further be noted that left rather than right inferior frontal activation distinguished our diagnostic groups. While we showed bilateral inferior activation patterns during response inhibition for all groups, no right-sided hypoactivation was observed in probands with ADHD. Previous studies in both healthy subjects and participants with ADHD have emphasized involvement of the right inferior frontal gyrus in response inhibition (
5,
58), although studies have also demonstrated functional involvement of the left inferior frontal gyrus in the response inhibition process (
59). We postulate that both inferior frontal nodes are involved in response inhibition and that lateralization may vary more between individuals than hitherto thought. This study strongly indicates that the left hemisphere should not be neglected and that future studies should be aimed at delineating the specific functional differences in response inhibition nodes between hemispheres.
The current study should be viewed in light of its strengths and limitations. A main strength was the large and well-documented sample. The unbalanced distributions of IQ and gender between diagnostic groups and scan sites were potential weaknesses of the current design.
To conclude, we demonstrated a distinction in neural activation patterns during response inhibition between adolescents with ADHD, their unaffected siblings, and healthy comparison subjects, indicating the familial nature of neural activation patterns underlying response inhibition in ADHD. Specifically, neural activation measures in superior frontal, inferior frontal, and temporal/parietal nodes of the response inhibition network showed hypoactivation patterns in line with the endophenotype model during failed but not successful response inhibition.