Bipolar disorder affects approximately 1% of the population worldwide, with little change in prevalence across generations (
1) despite substantial heritability (estimated to be between 59% and 85%) (
2,
3). This persistence appears paradoxical given the expected evolutionary impact of illnesses that affect brain function. For example, in schizophrenia, based on evidence of reduced fecundity before and after illness onset (
4), it is thought that persistence of the disorder at high prevalence is a function of relatively low selection pressure against any one liability-promoting mutation combined with the constant introduction of new liability-promoting mutations (
5), an inheritance model known as polygenic selection-mutation balance. However, the fertility disadvantage in bipolar disorder is less clear, with observations of reduced fecundity in clinic-derived samples (
6), minimal to no differences in population-based patient samples (
7,
8), and increased fertility in nonaffected siblings of bipolar probands (
7). One explanation, based on the anecdotal link between bipolar disorder and creativity (
9), posits that certain traits associated with liability to this syndrome may confer reproductive advantages. If liability to bipolar disorder is associated with creativity and other traits thought to be advantageous in mating selection, then the fertility disadvantage would be expected to be minimal or absent. In this study, our aim was to evaluate fitness-related features in individuals carrying liability to bipolar illness in order to examine possible mechanisms for an associated reproductive advantage in contrast to the known disadvantage associated with schizophrenia.
Evaluation of fitness-related traits in the unaffected family members of bipolar probands provides a basis for examining this model empirically. On average, such individuals carry higher degrees of liability (from both predisposing genes and environmental risk exposures) for disorder than individuals in the general population (
5). However, because unaffected family members do not have bipolar disorder themselves, measurement of liability-related traits is not complicated by secondary phenomena such as the effects of illness chronicity and exposure to psychotropic medications. Fitness-related traits of potential relevance include positive aspects of temperament (e.g., sociability and positive affect) and higher intellectual abilities. These traits have been theoretically and anecdotally related to bipolar disorder (
9–
13), are substantially heritable (
14,
15), and are linked to increased reproductive success in the general population (
16,
17). Such inquiry is aligned with growing interest in the study of positive virtues, character strengths, and associated well-being and mental health (
18). Wisdom and knowledge (including creativity and social intelligence) are among virtues shown to be associated with success and life satisfaction (
19).
If factors influencing liability to bipolar disorder confer greater fitness, the nonaffected first-degree relatives of bipolar probands would be expected to manifest higher levels of fitness-promoting phenotypes than members of the general population. If, on the other hand, there is no fitness-related advantage associated with bipolar illness, the nonaffected first-degree relatives would have either equivalent or lower levels of such traits compared with the general population, as has been observed in schizophrenia (
5).
Surprisingly few studies have addressed this question. Two early studies demonstrated increased prevalence of affective disorders (especially bipolar disorder) among prominent writers (
10) and individuals who achieved national recognition (
11), as well as among their first-degree relatives, compared with controls. Furthermore, trend-level enhanced creativity, defined by vocational and avocational activities, was observed in unaffected relatives of manic-depressive individuals compared with affected probands (
20). However, these studies suffered from several important limitations, including sampling flaws (e.g., selection of high-IQ eminent writers), the lack of modern, reliable clinical evaluations, and ambiguous measurement of constructs such as genius and creativity. More recent studies comparing first-degree relatives of bipolar patients with healthy samples on standardized tests of neurocognitive functioning have produced mixed results (
15,
21). Because these studies used hospital admission-based ascertainment methods, it is unclear whether the samples of bipolar probands and relatives were representative of the overall populations of such individuals. Several studies examining total population registries suggest an association between enhanced cognitive functioning and risk for bipolar disorder (
12,
13) and an overrepresentation in creative professions compared with controls (
22).
We employed a population-based sampling strategy to compare twin pairs discordant for bipolar disorder and demographically similar control twins without a lifetime history of bipolar disorder or schizophrenia on well-validated measures of neurocognitive functioning and temperament. Specifically, we tested whether the non-ill co-twins of bipolar probands showed enhanced levels of prosocial temperament characteristics and neurocognitive functioning relative to controls, as predicted by the reproductive fitness model. Alternatively, levels similar to or lower than those of controls would lend support to a polygenic selection-mutation balance model. Negative temperament was also assessed to explore the specificity of findings in relation to prosocial temperament. Parallel analyses were conducted in twin pairs discordant for schizophrenia for model comparison.
Method
The study protocol was reviewed and approved by the institutional review board of the University of California, Los Angeles, and the regional ethical review board at Karolinska Institute in Stockholm. All participants signed approved informed consent forms prior to participation.
Participants
The Swedish Twin Registry and Swedish National Patient Registry were linked to identify same-sex twin pairs born in Sweden between 1940 and 1985 containing at least one member with a hospital discharge diagnosis of schizophrenia or bipolar I disorder (see reference
3 for detailed recruitment procedures). This yielded a cohort of 562 potential probands (257 male and 305 female) (see Figure S1 in the
data supplement that accompanies the online edition of this article). Discordant monozygotic and dizygotic pairs were recruited randomly from this population, along with demographically balanced samples of control pairs of each zygosity. Twin pairs recruited as discordant in which the co-twin was determined to have an eligible diagnosis, and control pairs in which either co-twin was found to meet criteria for an eligible diagnosis, remained eligible and were reclassified accordingly.
The final sample consisted of 258 individuals. Participants’ demographic and clinical characteristics, along with information on zygosity, are presented in
Table 1. There were no differences between groups in age, sex, handedness, or years of education. There were no differences between co-twins and controls in lifetime medication status or lifetime axis I diagnoses except history of substance use disorders, for which schizophrenia co-twins had a greater incidence than bipolar co-twins and controls (χ
2=7.61, p=0.02). As expected, significant group differences were observed on symptom ratings, such that bipolar and schizophrenia probands had higher scores relative to co-twins and controls, who did not differ from each other.
Procedures
Clinical evaluation.
Diagnostic status was determined using clinical interview and medical register diagnostic history dating back to 1973. Each participant was interviewed by a psychiatrist using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (
23) and the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (
24). The determination of a lifetime history of psychosis in bipolar probands was based on information gathered from the psychosis module of the SCID, with psychosis defined as the occurrence of hallucinations and/or delusions during at least one affective episode. Thirty percent of bipolar probands (19 of 64) had a positive lifetime history of psychosis. All participants were rated using the Hamilton Depression Rating Scale (HAM-D) (
25), the Young Mania Rating Scale (YMRS) (
26), the Scale for the Assessment of Negative Symptoms (SANS) (
27), and the Scale for the Assessment of Positive Symptoms (SAPS) (
28).
Participation criteria.
Eligibility for inclusion as a proband was a consensus diagnosis of bipolar I disorder or schizophrenia/schizoaffective disorder. Exclusion criteria for all participants included a neurological disorder, history of significant head injury with loss of consciousness, mental retardation, substance use disorder within the previous 6 months, and inability to read or comprehend spoken and written Swedish. All proband participants were clinically stable (i.e., were outpatients living independently or residents of board and care homes) and were receiving medication and/or were in a period of remission. Healthy control twin pairs were screened to exclude individuals with a personal or family history of bipolar disorder or schizophrenia.
Zygosity.
DNA analysis determined zygosity following the procedure described by Hannelius et al. (
29) using a highly multiplexed panel of 47 single-nucleotide polymorphisms, including a sex-specific marker, to calculate percent of allele sharing between twins. Likelihood of zygosity for the genotyped twin samples was then calculated assuming a 1% prior genotyping error rate, which is expected to result in a false positive rate <0.01.
Temperament Measures
All participants completed a battery of well-established, psychometrically sound self-report questionnaires: the Barratt Impulsivity Scale, Version 11 (
30), a measure of attentional, motor, and nonplanning trait impulsiveness; the Zuckerman Sensation-Seeking Scale, Form II (
31), a measure of sensitivity to internal sensations; the Schizotypal Personality Questionnaire (
32), based on diagnostic criteria for schizotypal personality disorder; and the Temperament Evaluation of Memphis, Pisa, Paris, and San Diego–autoquestionnaire version, short-form (
33), based on temperamental variations in psychiatric patients and healthy volunteers.
Temperament scale creation.
Items from the instruments described above were used to derive two contrasting temperament scales indexing positive and negative temperament traits (see Table S1 in the online data supplement) in a procedure that combined theoretical and empirical approaches for optimal scale construction. First, exploratory principal-components analysis of the 133 original test items was conducted to extract the major unifying factors that categorized scale items. Results indicated four predominant factors, one characterized by elements of prosocial temperament and three comprising more typically “negative” temperament characteristics. A composite scale of negative temperament was formed, as negativity scales were highly conceptually related and correlated. Coefficient alpha was maximized by subtracting items with low correlations with scale totals. The final 24-item “positivity” scale is characterized by social ease, confidence, and assertiveness. It demonstrated acceptable internal consistency (Cronbach’s alpha=0.72). The final 28-item “negativity” scale is marked by depressiveness, anxiety, suspiciousness, and social withdrawal. It showed good internal consistency (Cronbach’s alpha=0.88). The two scales were moderately negatively correlated (r=−0.20, p<0.01).
Neuropsychological Assessment
A standard neuropsychological test battery was administered to all participants in a fixed order by a neuropsychologist. The battery included the following well-validated tests: the vocabulary and block design subtests of the Wechsler Adult Intelligence Scale–Revised (
34); the digit span, spatial span, and visual reproduction subtests of the Wechsler Memory Scale–Revised (
35); the phonemic and semantic verbal fluency tests (
36); the Trail Making Test, parts A and B (
37); the California Verbal Learning Test–Revised (
38); the divided attention task (
39); the verbal working memory task (
40); and the grooved pegboard test (
41). All of the measures were translated into Swedish and back-translated into English; the Swedish-language versions have been validated and used extensively in previous studies (e.g.,
42).
Neurocognitive domains.
An initial principal-components analysis of the 13 neuropsychological tests identified three predominant factors broadly characterized by 1) g factor; 2) high verbal performance (including measures of verbal fluency and verbal memory) and low visuospatial performance; and 3) attention/working memory and psychomotor processing speed. Construction of neurocognitive domains was based on this pattern of results and followed previous efforts to categorize neuropsychological tests into domains (e.g.,
43,
44). Individual scores were z-transformed relative to controls and averaged across relevant tasks to form five domains (estimated IQ: vocabulary, block design; nonverbal learning and memory: spatial span, visual reproduction-delayed; verbal learning and fluency: phonemic and semantic verbal fluency, California Verbal Learning Test [learning slope, long-delay free recall]; attention and working memory: digit span, Trail Making Test part B, divided attention task, verbal working memory task; psychomotor processing speed: Trail Making Test part A, grooved pegboard test).
Statistical Analysis
Mixed-model analyses of variance with repeated measures were conducted to compare temperament and cognitive scores of bipolar and schizophrenia probands, their unaffected co-twins, and controls. Separate analyses were conducted for temperament scales and neurocognitive domains. Data were analyzed using PROC MIXED in SAS, version 9.2 (SAS Institute, Cary, N.C.). A multivariate approach was used to account for correlation among repeated effects (i.e., temperament scales, neurocognitive factors). To account for the clustered nature of the twin data, subjects were treated as individuals nested within pairs, and the data were modeled with an unstructured variance-covariance matrix form, allowing for unique variance within each group and covariance within each twin pair. A multivariate approach was used to guard against type I errors, and simple effects were examined only when multivariate effects were significant. Age and sex were entered into the models as covariates. Interaction terms for age and sex were not significant and hence were dropped from the models, and data were collapsed across zygosity for primary analyses.
Tests of linear trends on genetic loading for bipolar disorder were conducted contrasting monozygotic co-twins, dizygotic co-twins, and controls. All analyses measured significance at the 0.05 level (two-tailed).
Results
Tests of Sample Representativeness
The studied probands were comparable to the remainder of the twin proband population in sex distribution, age at first hospital admission, and number of hospital admissions. However, studied probands were younger on average than the remainder of the twin proband population (proband participants: mean=49.24 years, SD=10.44; proband nonparticipants: mean=53.65 years, SD=10.22; t=3.33, df=241, p<0.01). To control for this potential bias, we included age as a covariate and matched groups on age. There were no interactions of age with any other effects, and a median split on age produced equivalent results in both the younger and older samples, suggesting that the observed age differences cannot account for the pattern of results described below.
Temperament Analyses
There was a significant interaction between group and temperament scale (F=16.15, df=4, 252, p<0.01), with groups differing significantly on the positivity scale (F=6.32, df=4, 104, p<0.01) and the negativity scale (F=12.71, df=4, 104, p<0.01). Results are summarized in
Figure 1. Bipolar co-twins scored significantly higher on the positivity scale than bipolar probands (t=2.08, df=104, p<0.05) and controls (t=−2.89, df=104, p<0.01). Bipolar probands were indistinguishable from controls on this scale. In contrast, bipolar co-twins scored significantly lower than bipolar probands on the negativity scale (t=−3.48, df=104, p<0.01). Their scores were not significantly lower than those of controls. Scores of bipolar probands were elevated on this measure compared with both other groups (co-twins: t=−3.48, df=104, p<0.01; controls: t=−2.85, df=104, p<0.01). These findings were equivalent when clinical ratings of mania symptoms (total score on the YMRS) were added to the model. There was also a significant main effect of age on scale scores (F=14.85, df=1, 252, p<0.01). A 1-year increase in age was associated with a decrease of 0.08 in overall mean scale score (t=−3.85, df=252, p<0.01). This relationship did not vary depending on group or scale.
In contrast, schizophrenia co-twins exhibited a similar, although attenuated, pattern of results compared with their proband counterparts. Linear trend analysis revealed evidence of a stepwise pattern in which schizophrenia probands showed significantly lower positivity scores than controls, with co-twins in-between (p<0.05) (see
Figure 1). On the negativity scale, schizophrenia probands had higher scores than their co-twins (t=5.26, df=104, p<0.01) and controls (t=4.86, df=104, p<0.01), and co-twins were indistinguishable from controls.
Neurocognitive Analyses
There was a significant interaction between group and neurocognitive domain (F=4.11, df=16, 1011, p<0.01). There was also a significant main effect of age on neuropsychological performance (F=23.34, df=1, 1011, p<0.01); this relationship did not vary by group or neurocognitive factor. Specifically, a 1-year increase in age was associated with a decrease of 0.02 in overall z-transformed mean score (t=−4.83, df=1011, p<0.01).
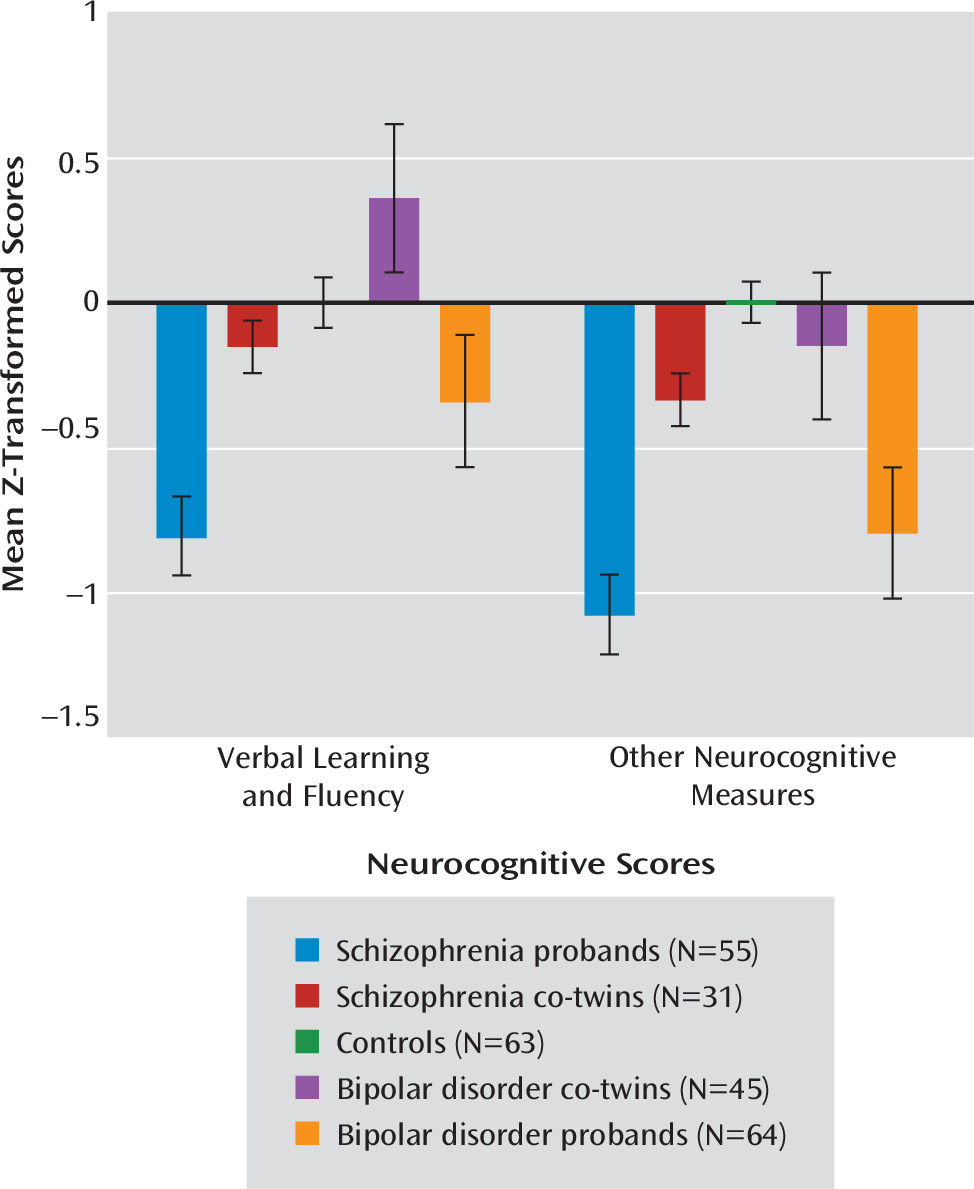
Tests of simple effects demonstrated significant group differences on all domains (estimated IQ: F=9.02, df=4, 104, p<0.01; nonverbal learning and memory: F=10.95, df=4, 104, p<0.01; verbal learning and fluency: F=14.21, df=4, 104, p<0.01; attention and working memory: F=7.14, df=4, 104, p<0.01; psychomotor processing speed: F=10.64, df=4, 104, p<0.01). Bipolar probands showed deficits across all neurocognitive factors relative to their unaffected co-twins (estimated IQ: t=3.47, df=104, p<0.01; nonverbal learning and memory: t=4.22, df=104, p<0.01; verbal learning and fluency: t=4.81, df=104, p<0.01; attention and working memory: t=2.88, df=104, p<0.01; psychomotor processing speed: t=3.19, df=104, p<0.01) and controls (estimated IQ: t=3.16, p<0.01; nonverbal learning and memory: t=4.22, df=104, p<0.01; verbal learning and fluency: t=2.43, df=104, p=0.02; attention and working memory: t=3.13, df=104, p<0.01; psychomotor processing speed: t=3.79, df=104, p<0.01). These deficits were most pronounced in nonverbal learning and memory and psychomotor processing speed. Bipolar co-twins scored significantly higher on the verbal learning and fluency domain relative to controls (t=−2.01, df=104, p<0.05) (
Figure 2). For all other factors, bipolar co-twins were indistinguishable from controls. These findings were equivalent when YMRS total score was included in the model, indicating that effects were not attributable to clinical ratings of mania symptoms.
In contrast, schizophrenia co-twins exhibited a pattern of attenuated neurocognitive impairments relative to their schizophrenia twins. Schizophrenia probands showed deficits across all domains compared with their unaffected co-twins (estimated IQ: t=−4.05, df=104, p<0.01; nonverbal learning and memory: t=−3.93, df=104, p<0.01; verbal learning and fluency: t=−4.09, df=104, p<0.01; attention and working memory: t=−3.10, df=104, p<0.01; psychomotor processing speed: t=−3.59, df=104, p<0.01) and controls (estimated IQ: t=−3.89, df=104, p<0.01; nonverbal learning and memory: t=−3.78, df=104, p<0.01; verbal learning and fluency: t=−4.84, df=104, p<0.01; attention and working memory: t=−4.22, df=104, p<0.01; psychomotor processing speed: t=−5.48, df=104, p<0.01). Linear trend analysis revealed a significant effect of liability for schizophrenia for each neurocognitive domain (p values <0.01) whereby schizophrenia probands performed worse than their unaffected co-twins, who in turn performed worse than controls.
Neurocognitive results were equivalent when education level (in years) was included in the model as a covariate.
Exploratory Analyses
To further characterize positive temperament and verbal ability, heritability estimates were calculated using Falconer’s formula, which roughly approximates the proportion of total phenotypic variation attributable to genetic effects. The analysis comprised a total of 30 control twin pairs, 16 of them monozygotic and 14 of them same-sex dizygotic; three singletons were excluded. Proband twin pairs were excluded from analyses to minimize the impact of illness state on scores and covariance structures. The heritability of the temperament positivity scale was estimated at 0.44 (monozygotic: r=0.61; dizygotic: r=0.39). Estimated heritability of the verbal learning and fluency domain was 0.70 (monozygotic: r=0.69; dizygotic: r=0.34). This pattern of correlations suggests substantial additive genetic effects for both domains as well as a common environmental effect for the positivity scale, although our ability to further parse the relative genetic and environmental contributions is limited by the sample size.
Linear trend analysis comparing controls with dizygotic and monozygotic bipolar co-twins revealed a significant linear effect for the temperament positivity scale (F=5.59, df=1, 29, p<0.03) and the verbal learning and fluency domain (F=4.32, df=1, 29, p<0.05). Although post hoc tests revealed significant differences between controls and dizygotic bipolar co-twins (positivity: t=−2.86, df=29, p<0.01; verbal: t=−2.92, df=29, p<0.01), monozygotic and dizygotic co-twins did not differ from each other on either temperament positivity or verbal learning and fluency, possibly because of lack of power due to the small cell sizes of co-twins split by zygosity. Furthermore, there were no differences in neurocognitive or temperament measures between co-twins of bipolar probands with and without a lifetime history of psychosis. However, these analyses were underpowered because of the small sample of co-twins of bipolar probands with a history of psychosis. The majority of bipolar probands with a history of psychosis were concordant twins, leaving only one-third of nonaffected co-twins of bipolar probands with such a history.
Discussion
The persistence of severe mental illness is a paradox that has long puzzled evolutionary psychologists and psychiatric geneticists. We sought in this study to evaluate the presence of key fitness-related traits (temperament and neurocognitive abilities) in nonaffected twins of bipolar disorder probands to evaluate a model of reproductive fitness in contrast to a selection-mutation balancing model of mental disorder liability. The former model posits that susceptibility alleles confer adaptive benefits under certain circumstances or below some threshold for disorder. In support of a reproductive fitness model, we found that certain temperament and neurocognitive benefits appear to be associated with liability for bipolar disorder. Specifically, bipolar co-twins had elevated scores on a “positivity” temperament scale, indicating traits of social ease, confidence, and assertiveness, as well as superior neurocognitive performance on tests of verbal learning and fluency relative to healthy subjects. This study provides evidence that increases in liability for bipolar disorder that remain below some clinical threshold appear to be associated with fitness-promoting features such as sociability and verbal facility. Such findings are consistent with evidence of minimal to no evolutionary impact of bipolar disorder (
7,
8) in contrast to the severe fertility disadvantages observed in schizophrenia (
4).
Personality and temperament traits occurring at the extreme ends of normalized dimensions have been associated with risk for psychiatric disorders and, by extension, reduced reproductive fitness (e.g., “difficult” temperament [
45], neuroticism, and mood lability [
46]). Our findings of elevated pessimism, suspiciousness, and asociality among both proband samples support a close association between negative personality characteristics and illness state. Conversely, the personality traits we examined here, encompassing aspects of prosociality and positive affect, have relevance to mate selection and reproductive success. In general, individuals who tend to be more verbal, outgoing and sociable, optimistic, and psychologically resilient may be viewed as more desirable partners (
47,
48).
In terms of neurocognition, previous work demonstrated that poor verbal learning and memory was associated with functional impairment in euthymic bipolar subjects (
49), suggesting that deficits in this particular domain may be related to impaired role functioning and thereby decreased fitness. On the other hand, in the general population, higher intelligence is associated with lower mortality rates (
50) and increased reproductive fitness (
51,
52). Whether such benefits can be attributed to specific domains of intelligence, such as verbal ability, is not known. Our findings suggest that the characteristic personality and neurocognitive profiles evident in bipolar co-twins due to the presence of susceptibility factors may indeed confer increased reproductive fitness while remaining below threshold for disorder. The observed increases in fitness-promoting features in nonaffected bipolar relatives dovetail with evidence of increased fertility in healthy sisters of bipolar probands (
7), suggesting enhanced fitness in nonaffected individuals carrying bipolar liability factors.
The performance benefits observed in bipolar co-twins appear unique to specific personality and neurocognitive domains, rather than representative of a ubiquitous advantage. Bipolar co-twins did not show reduced levels of negative temperament relative to controls, and the two scales were only moderately correlated (r=−0.20). Similarly, scores of bipolar co-twins were equivalent to those of controls on other neurocognitive dimensions, with selected strengths in the verbal domain. These findings stand in contrast to previous neuropsychological studies demonstrating support for candidate neurocognitive endophenotypes for bipolar disorder (
15,
21), with nonaffected first-degree relatives showing impairments relative to healthy controls in the domains of executive functioning, verbal memory, and sustained attention. However, those studies used clinic-derived samples, which are heavily weighted toward more severe cases. The key advantage of a population-based sampling approach, as employed here, is confidence that the studied probands are representative of the broader population of probands—and, by extension, the studied nonaffected co-twins of bipolar probands are likely to be representative. Moreover, one previous study demonstrated intact verbal learning and memory in first-degree relatives of patients with bipolar I disorder despite impairments in executive functioning relative to controls (
53). Furthermore, a recent cohort study of over 1 million men suggested that verbal aspects of intelligence may serve as a risk factor for pure forms of bipolar disorder (i.e., without psychiatric comorbidities [
54]). Our findings here suggest that the benefits associated with liability for bipolar disorder may be unique to the domain of verbal skills.
The findings for bipolar co-twins are contrasted with those for schizophrenia co-twins, who did not exhibit such benefits but rather showed deficits intermediate between controls and probands. Schizophrenia co-twins showed lower levels of “positivity” and more generalized reductions in neurocognitive functioning relative to controls, although not as low or impaired as schizophrenia probands. Co-twins of schizophrenia probands had deficits on the same traits on which co-twins of bipolar patients showed advantaged or equivalent performance relative to controls, suggesting segregation at the endophenotypic level between these two syndromes, at least with respect to temperament and neurocognitive functioning. The neurocognitive domains of nonverbal learning and memory, attention and working memory, and psychomotor processing speed appear to be particularly robust endophenotypic markers of schizophrenia, consistent with previous studies (
5). No neurocognitive impairment was specific to bipolar disorder, as both proband groups tended to show performance decrements, indicating that illness manifestation is associated with impairments in both syndromes. Taken together, the findings in schizophrenia implicate a polygenic selection-mutation balance, whereby genetic persistence of illness appears to hinge on the maintenance of many small-effect genetic variants in the population, which may be common, rare, inherited, or de novo, but against which there is relatively low selection pressure. This highlights the possibility of an inherent difference between bipolar disorder and schizophrenia and suggests that a single evolutionary model to explain the presence of all forms of mental illness in the population is unlikely.
A possible limitation of this study is the slightly younger proband samples relative to the remainder of the proband population. That we recruited fewer older participants than is representative of the general population suggests that our results may have an ascertainment bias toward younger proband twin pairs. It is possible that an age difference emerged because older probands were more likely to decline study participation because of age-related health issues or travel difficulties, or because they could not be reached because of illness or other factors. However, we controlled for this potential bias by matching groups on age, and there were no interactions between age and group on neurocognitive or temperament scores. Secondary analyses bifurcating groups on age showed equivalent effects. Therefore, the characterized age differences cannot account for the observed pattern in the present findings. Additionally, it is possible that nonaffected relatives of more severe cases (in terms of genetic loading, history of psychosis, shared genetic liability with schizophrenia, or clinical severity) would not exhibit fitness-promoting features but rather performance decrements, consistent with initial findings of reduced fecundity from clinic-derived bipolar samples (
6). Future studies should ensure adequate power to investigate heterogeneity in bipolar disorder.
To our knowledge, this is the first systematic study to indicate a possible familial compensatory advantage to bipolar illness in discordant twin pairs. These findings contribute to mounting evidence linking creativity and enhanced cognitive functioning and risk for bipolar disorder (
9–
13,
20,
22) and may inform models of mechanisms underpinning this association. That liability for bipolar disorder may confer temperament and neurocognitive benefits could provide one explanation for the genetic persistence of this illness.