The STRIDE Weight Loss and Lifestyle Intervention for Individuals Taking Antipsychotic Medications: A Randomized Trial
Abstract
Objectives:
Method:
Results:
Conclusions:
Method
Study Design
| Increasing awareness through monitoring: diet, physical activity, and sleep |
|---|
| Creating personalized diet and physical activity plans |
| Reducing calories |
| Reducing portion sizes, identifying and choosing alternative foods, modifying meals |
| Increasing consumption of fruits, vegetables, fiber, and low-fat dairy products |
| Increasing physical activity |
| Developing action plans for high-risk eating situations |
| Graphing progress and making adjustments |
| Addressing mental health effects on lifestyle-change efforts |
Settings
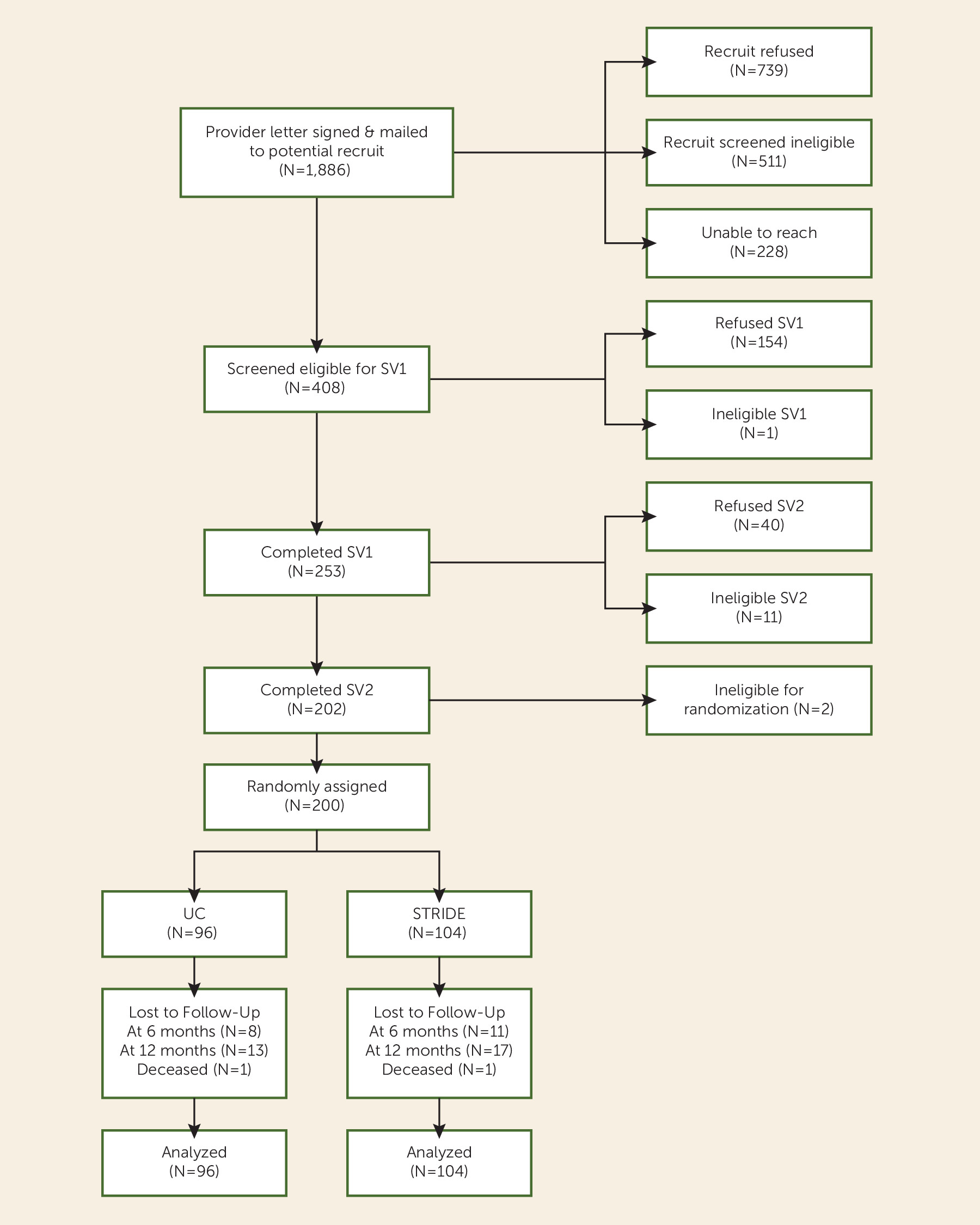
Recruitment, Screening, and Randomization
Intervention
Initial intervention.
Maintenance intervention.
Assessment, Data Collection, and Measurement
Statistical Analyses
Sample Size
Results
Participants
| Characteristic | Total (N=200) | Intervention Group (N=104) | Control Group (N=96) | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 47.2 | 10.6 | 46.2 | 11.4 | 48.3 | 9.7 |
| Weight (kg) | 107.7 | 25.1 | 108.6 | 27.2 | 106.6 | 22.7 |
| Body mass index | 38.3 | 8.3 | 38.3 | 9.1 | 38.2 | 7.3 |
| Female waist circumference (cm) | 114.5 | 19.2 | 114.6 | 20.5 | 114.4 | 17.7 |
| Male waist circumference (cm) | 112.4 | 17.5 | 113.8 | 19.6 | 110.9 | 15.1 |
| Systolic blood pressure (mmHg) | 119.2 | 14.7 | 117.5 | 14.2 | 121.0 | 15.2 |
| Diastolic blood pressure (mmHg) | 79.4 | 10.1 | 78.5 | 9.7 | 80.4 | 10.5 |
| Fasting triglycerides (mg/dL) | 188.0 | 138.6 | 188.0 | 130.3 | 188.0 | 147.8 |
| Fasting low-density lipoprotein (mg/dL) | 101.4 | 32.9 | 101.4 | 31.3 | 101.4 | 34.7 |
| Fasting high-density lipoprotein (mg/dL) | 45.8 | 12.7 | 46.6 | 14.0 | 45.0 | 11.0 |
| Fasting total cholesterol (mg/dL) | 181.6 | 39.7 | 183.2 | 38.7 | 179.9 | 40.9 |
| Fasting plasma glucose (mg/dL) | 108.9 | 32.5 | 107.6 | 31.2 | 110.3 | 34.1 |
| Fasting insulin (μU/mL) | 13.0 | 11.9 | 11.2 | 7.8 | 15.1 | 15.0 |
| Number of psychiatric medications | 3.2 | 1.5 | 3.1 | 1.4 | 3.3 | 1.5 |
| Modified Colorado Symptom Index score | 19.3 | 11.4 | 18.3 | 11.2 | 20.4 | 11.6 |
| Behavior and Symptom Identification Scale-24 item score | 1.37 | 0.68 | 1.29 | 0.70 | 1.47 | 0.64 |
| Short-Form 36 Health Survey, Version 2.0, general health item score | 42.08 | 9.99 | 42.79 | 10.94 | 41.33 | 8.84 |
| N | % | N | % | N | % | |
| Female sex | 144 | 72.0 | 75 | 72.1 | 69 | 71.9 |
| Race/ethnicity | ||||||
| White | 174 | 87.7 | 90 | 88.2 | 81 | 87.1 |
| Nonwhite | 26 | 12.3 | 12 | 11.8 | 12 | 12.9 |
| Hispanic | 4 | 2.0 | 3 | 2.9 | 1 | 1.1 |
| Education level | ||||||
| Less than high school diploma/GED | 15 | 7.5 | 6 | 5.8 | 9 | 9.4 |
| High school graduate/GED | 46 | 23.0 | 26 | 25.0 | 20 | 20.8 |
| Some college | 87 | 43.5 | 44 | 42.3 | 43 | 44.8 |
| College graduate | 52 | 26.0 | 28 | 26.9 | 24 | 25.0 |
| Never married | 57 | 28.5 | 37 | 35.6 | 20 | 20.8 |
| Currently working | 59 | 29.5 | 27 | 26.0 | 32 | 33.3 |
| Receiving disability income | 90 | 45 | 46 | 44.2 | 44 | 45.8 |
| Individual monthly income | ||||||
| <$500 | 33 | 16.5 | 20 | 20.0 | 13 | 13.7 |
| $500–$1,000 | 66 | 33.8 | 35 | 35.0 | 31 | 32.6 |
| $1,000–$1,499 | 36 | 18.5 | 20 | 20.0 | 16 | 16.8 |
| $1,500–$1,999 | 17 | 8.7 | 6 | 6.0 | 11 | 11.6 |
| $2,000–$2,499 | 18 | 9.2 | 8 | 8.0 | 10 | 10.5 |
| ≥$2,500 | 25 | 12.9 | 11 | 11.0 | 14 | 14.8 |
| Mental health diagnoses (from medical records) | ||||||
| Schizophrenia spectrum disorder | 58 | 29.0 | 31 | 29.8 | 27 | 28.1 |
| Bipolar disorder or affective psychosis | 138 | 69.0 | 71 | 68.2 | 67 | 69.8 |
| Posttraumatic stress disorder | 4 | 2.0 | 2 | 1.9 | 2 | 2.1 |
| Current medications | ||||||
| Blood pressure medications | 59 | 29.5 | 30 | 28.8 | 29 | 30.2 |
| Diabetes medications | 30 | 15.0 | 14 | 13.5 | 16 | 16.7 |
| Cholesterol medications | 49 | 24.5 | 27 | 26.0 | 22 | 22.9 |
| Atypical antipsychotic medications | 182 | 91.0 | 95 | 91.3 | 87 | 90.6 |
| Lithium or anticonvulsant medications | 97 | 48.5 | 51 | 49.0 | 46 | 47.9 |
| Antidepressant medications | 33 | 16.5 | 15 | 14.4 | 18 | 18.8 |
| Current psychiatric medications classified according to weight loss/gain profilea | ||||||
| ≥1 slight/moderate weight loss | 77 | 38.5 | 39 | 37.5 | 38 | 39.6 |
| ≥1 weight neutral | 168 | 84.0 | 87 | 83.7 | 81 | 84.4 |
| ≥1 slight/moderate weight gain | 21 | 10.5 | 10 | 9.6 | 11 | 11.5 |
| ≥1 severe weight gain | 128 | 64.0 | 68 | 65.4 | 60 | 62.5 |
Study Retention and Intervention Attendance
Analyses
| Variable | Δ Baseline–6 Months | Δ 6 Months–12 Months | Δ Baseline–12 Months | pb | |||
|---|---|---|---|---|---|---|---|
| Coefficienta | 95% CI | Coefficienta | 95% CI | Coefficienta | 95% CI | ||
| Weight (kg) | –4.37 | –6.96 to –1.78 | 1.77 | –0.87 to 4.40 | –2.60 | –5.14 to –0.07 | 0.004 |
| Body mass index (kg/m2) | –1.55 | –2.47 to –0.63 | 0.58 | –0.35 to 1.50 | –0.97 | –1.88 to –0.06 | 0.004 |
| Systolic blood pressure (mmHg) | –1.60 | –5.21 to 2.02 | 1.68 | –1.91 to 5.27 | 0.09 | –3.36 to 3.53 | 0.60 |
| Diastolic blood pressure (mmHg) | –1.21 | –3.58 to 1.17 | 0.82 | –1.59 to 3.23 | –0.38 | –2.89 to 2.12 | 0.59 |
| Fasting glucose (mg/dL) | –0.02 | –0.08 to 0.05 | –0.08 | –0.14 to –0.01 | –0.09 | –0.16 to –0.02 | 0.02 |
| Fasting insulin (μU/mL) | 0.11 | –0.09 to 0.31 | –0.12 | –0.58 to 0.34 | –0.01 | –0.46 to 0.43 | 0.56 |
| Homeostasis model assessment-insulin resistance indexc | 0.09 | –0.12 to 0.30 | –0.15 | –0.37 to 0.07 | –0.24 | –0.48 to 0.01 | 0.17 |
| Diabetes riskd | 0.17 | –1.55 to 1.89 | –1.56 | –3.39 to 0.26 | –1.39 | –3.09 to 0.31 | 0.17 |
| Fasting triglycerides (mg/dL) | 3.72 | –21.57 to 29.01 | 0.67 | –21.52 to 22.86 | 4.39 | –24.18 to 32.96 | 0.95 |
| Fasting low-density lipoprotein (mg/dL) | –1.65 | –8.52 to 5.22 | 1.68 | –5.25 to 8.61 | 0.03 | –7.58 to 7.64 | 0.85 |
| Fasting high-density lipoprotein (mg/dL) | 1.23 | –0.70 to 3.16 | 1.05 | –1.14 to 3.24 | 2.28 | –0.14 to 1.05 | 0.17 |
Primary outcomes.
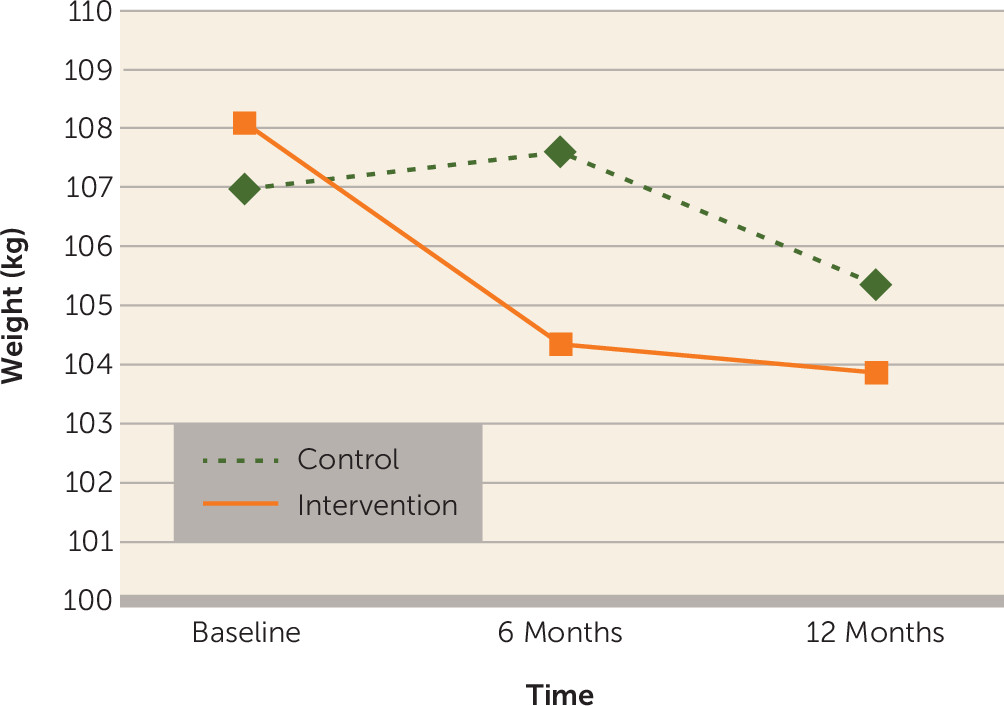

Secondary outcomes.
Acute service use and adverse events.
Discussion
Patient Perspectives
Limitations and Opportunities
Conclusions
Acknowledgments
Footnote
Supplementary Material
- View/Download
- 190.00 KB
- View/Download
- 25.56 KB
- View/Download
- 17.54 KB
- View/Download
- 190.00 KB
- View/Download
- 25.56 KB
- View/Download
- 17.54 KB
References
Information & Authors
Information
Published In
History
Authors
Competing Interests
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

