We address four questions about the IQ-schizophrenia relationship, the first two of which attempt to refine earlier findings. First, can we confirm previous findings of an association of IQ and schizophrenia risk and rule out the possibility that this association reflects prodromal effects of an impending schizophrenic illness? Second, can we clarify, in more detail than previously possible, the nature of the “dose-response” relationship between IQ and risk for schizophrenia? Is the association linear or nonlinear, and if nonlinear, is it stronger at lower or at higher IQs (
3)? Furthermore, is the association monotonic, so that the lowest risk for schizophrenia is seen with the highest IQ? Or, as suggested by some previous analyses (
3), is the function U-shaped, with the lowest risk at moderately high IQs and the highest IQ scores associated with a greater risk of illness?
We then examine two important questions about the IQ-schizophrenia association that, to our knowledge, have not been previously addressed. First, using a co-relative design (which selects pairs of relatives with discrepant risk factor exposures and examines their difference in a disease outcome), can we determine the degree to which the association between IQ and schizophrenia arises from shared familial/genetic factors or from a possible causal relationship? Second, what is the etiologic relationship between genetic liability to schizophrenia and premorbid IQ? Are these two risk factors additive in their effect, or do they interact? If they interact, does a high genetic liability to schizophrenia have a greater impact on those with low, or those with high IQ?
Method
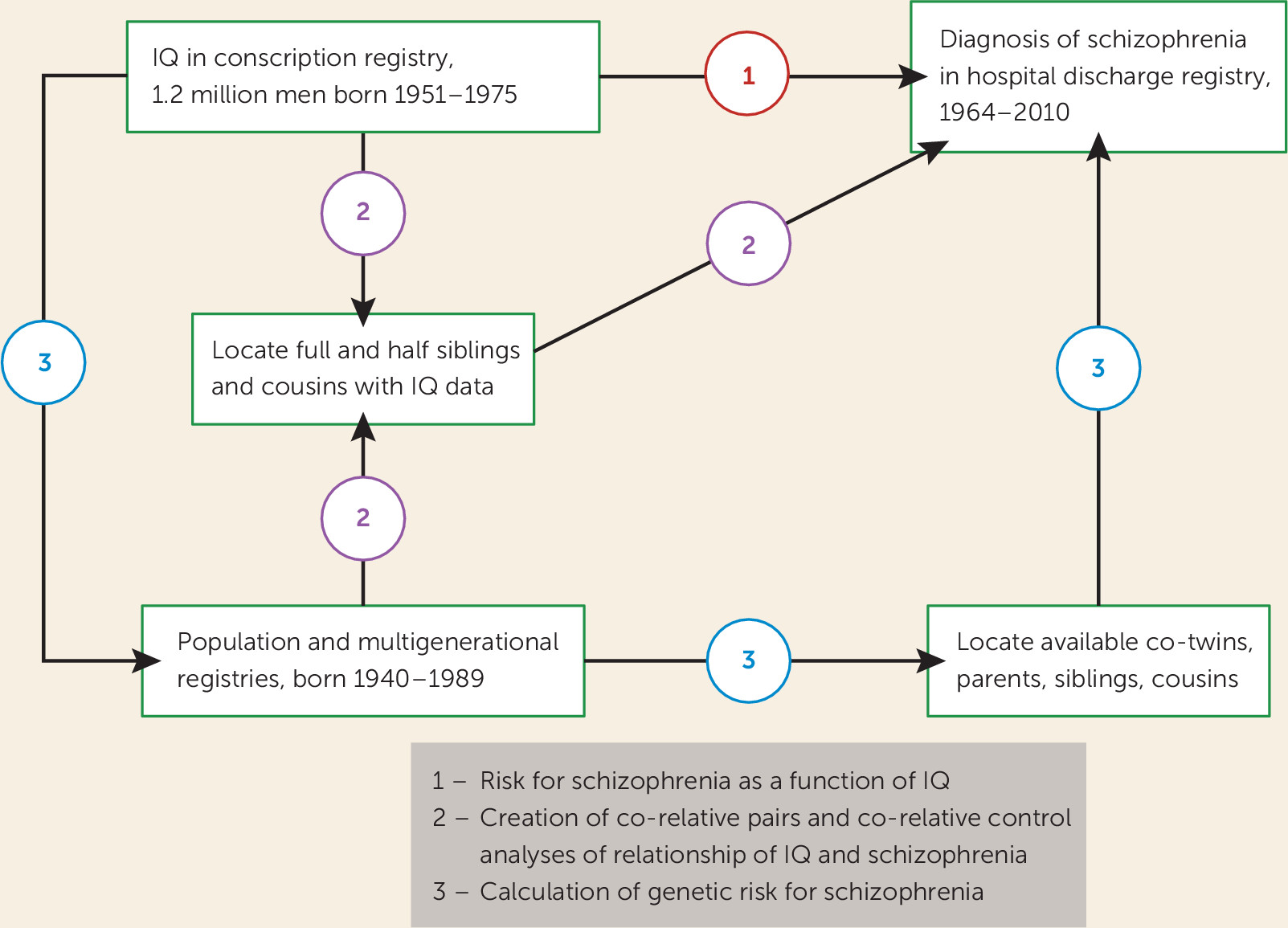
We used data from multiple Swedish nationwide registries linked by the unique individual Swedish 10-digit personal identification number assigned at birth or immigration to all Swedish residents; identification numbers were replaced by random numbers to preserve confidentiality. As illustrated in
Figure 1, the analyses reported here were largely based on four Swedish registries: the Military Conscription Register, which includes cognitive assessments for nearly all 18-year-old men in Sweden; the Total Population Register, containing annual data on family status; the Multi-Generation Register, which provides information on family relations; and the Swedish Hospital Discharge Register, which contains data on all hospitalizations in Sweden since 1964. We also used data from the Swedish Mortality Register to calculate the follow-up time in our hazard models. The Swedish military service conscription examination involves a full medical assessment that includes cognitive function (IQ), measured by four subtests representing logical, spatial, verbal, and technical abilities. During the years covered by this study, this examination was required by law. Among all men born in Sweden, only those with serious medical conditions or disabilities were excused from the conscription examination (∼4.2% of individuals). The global IQ score, derived from a summation of the four subtests, was standardized to give a Gaussian-distributed score between 1 and 9, which we translated into units of the Wechsler Adult Intelligence Scale with a mean of 100 and a standard deviation of 15.
Schizophrenia was defined in the Swedish Hospital Discharge Register as ICD8 codes 295.1, 295.2, 295.3, 295.5, and 295.6; ICD9 codes 295B, 295C, 295D, 295G, and 295X; and ICD10 codes F20.0, F20.1, F20.2, F20.3, F20.5, F20.8, and F20.9. Nonaffective psychosis was defined as ICD8 codes 295, 297, 298.2, 298.3, and 298.9; ICD9 codes 295, 297, 298C, 298E, 298W, and 298X; and ICD10 code F2. The ratio of nonaffective psychosis to schizophrenia cases in our sample was 2.14:1. We obtained ethical approval for this study from the Regional Ethical Review Board of Lund University.
The database began with all male individuals in the Swedish population born between 1951 and 1975 who were registered in the Military Conscription Register, a total of 1,324,117 individuals. For 85,432 of these, no information was available for the IQ variable; 33,376 were missing data for the conscription examination; and 326 had a registration of schizophrenia prior to their conscription examination. In total, we investigated 1,204,983 male individuals, representing 87.2% of all male individuals born in Sweden between 1951 and 1975 who survived to age 18.
We used Cox proportional hazards models to investigate the future risk for schizophrenia in individuals as a function of their IQ score. Robust standard errors were used to adjust the 95% confidence intervals, as we had several individuals from the same family. Follow-up time in years was measured from year of conscription examination until year of first registration for schizophrenia, death, emigration, or end of follow-up (year 2010), whichever came first. In all models, we investigated the proportionality assumption. In a second step, we wanted to compare the results from the entire population with the results from a co-relative design. By means of the Swedish Multi-Generation Register, we identified all full-sibling, half-sibling, and first-cousin pairs. Using stratified Cox proportional hazards models, we performed three analyses on all full-sibling, all half-sibling, and all first-cousin pairs that did not have the same IQ score from the conscription registry, with a separate stratum for each relative pair. The co-relative design allowed us to contrast the rates of schizophrenia in relatives with different levels of IQ. The stratified Cox proportional hazards models provide a hazard ratio for IQ that is adjusted for the familial cluster and therefore accounts for an array of shared genetic and environmental factors.
To investigate the etiologic relationship between genetic liability to schizophrenia and IQ, we calculated a genetic liability score for schizophrenia. First, we calculated the morbid risk for schizophrenia for the population born between 1940 and 1989 using an abridged Weinberg age correction (
4). We defined three age groups as the first, the middle two (second and third), and the fourth quartiles of the age distribution of first schizophrenia registration: <28 years, weighted 0; 28–42 years, weighted 0.5; and >42 years, weighted 1. Next, we performed a logistic regression analysis based on information from the Swedish population born between 1940 and 1989 (N=5,646,776) and their relatives (monozygotic twins, dizygotic twins, full siblings, half siblings, mother, father, and cousins). The model outcome variable was schizophrenia, and the predictor variable was the morbid risk of schizophrenia among the different relative types. The resulting beta weights for the genetic liability score, which followed quantitative genetic expectations, are listed in Table S1 in the
data supplement that accompanies the online edition of this article. We did not use standard family history scores (
5) because they pool relatives, thereby ignoring the large impact on risk of schizophrenia in specific relatives resulting from fitness effects (
6,
7). We generated the beta weights in the same sample to which we applied them. While this traditionally might result in “overfitting,” this is a minimal concern when the entire population is being assessed. This model produced a predicted probability for schizophrenia ranging between 0.36% and 79%, termed the genetic liability score. We repeated this approach to assess genetic liability to nonaffective psychosis (see Table S2 in the online
data supplement).
We sought to examine the interaction between IQ and genetic liability on risk for schizophrenia. Along with Rothman et al. (
8), we favor a risk difference over a risk ratio approach to this question because it adopts a public health perspective, asking whether more cases of illness will arise when individuals at low versus high disease liability from one factor (genetic risk) are exposed to low versus high levels of another factor (IQ) (
9). Therefore, we analyzed interactions using an identity link function. In the first model, we estimated the interaction between IQ and genetic liability using the identity link. We then sought to investigate whether this interaction was driven by between-family or within-family IQ effects. Within each set of full siblings, we calculated the between and the within value of IQ (the between value is the mean value for each set of full siblings, and the within value is each sibling’s deviation from the mean value in the sibling set). In these models, the genetic liability was averaged over all siblings within each set of siblings. We then performed a regression model using the identity link and robust standard errors, with the genetic liability score, the between-sibling-set IQ score, the within-sibling-set IQ score, and the two interaction terms: one with the between IQ score and the genetic liability score, and one with the within IQ score and the genetic liability score. We could then see if the interaction effect was due to between or within IQ effects, as the genetic liability score was held constant for all siblings within a sibling set. All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, N.C.).
Discussion
We sought in this study to address, in a nearly complete sample of Swedish males born between 1951 and 1975, four questions about the relationship between IQ as assessed in late adolescence and subsequent risk for schizophrenia. First, we sought to replicate previous findings of an association between IQ and future risk for schizophrenia. A linear hazard ratio model showed an increase in risk of onset of 3.8% per 1-point decrease in IQ. This estimate is nearly identical to the estimate of 3.7% obtained from the most recent meta-analysis (
3). We also examined whether this association declined if we began our follow-up 5 years after IQ assessment, thereby eliminating most schizophrenia cases that would have been in a prodromal stage at testing. Congruent with a previous Finnish study that examined a 1-year “blocked out” interval (
10), we found no change in the association with our delayed follow-up period. The observed IQ-schizophrenia association does not, to any appreciable degree, appear to result from declines in intelligence in individuals undergoing an insidious onset of schizophrenia at the time of testing.
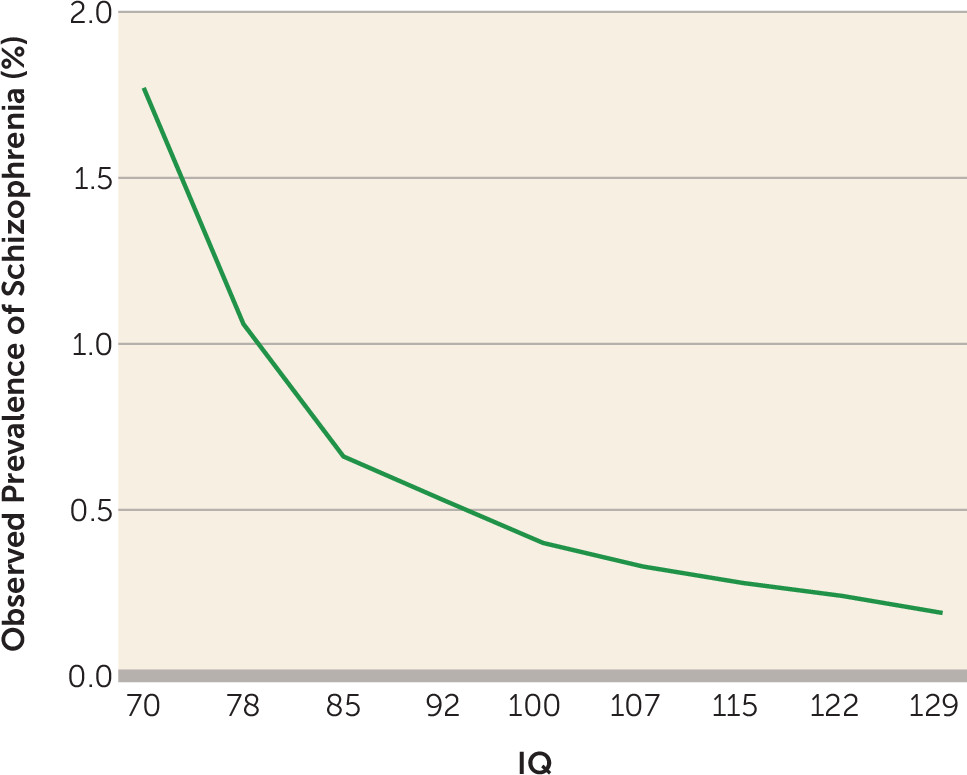
Our second goal was to clarify, with our large sample, the nature of the “dose-response” relationship between IQ and risk for schizophrenia. Inspection of the raw data (
Figure 2) revealed a monotonic relationship, beginning with a relatively steep slope in the low IQ range and then a decline in slope as IQ increased. Model fitting, consistent with results of the recent meta-analysis (
3), verified these impressions, showing both a significant linear and a significant quadratic function.
In an examination of the five strongest previous studies of the IQ-schizophrenia relationship, three of the studies showed a greater risk of illness in individuals at the highest IQ levels compared with those in the moderately high IQ levels (∼120) (
3). A Finnish study found substantial enrichment for cases of schizophrenia among those with the highest level of academic achievement in high school (
11). Such findings suggest a relationship between schizophrenia and “genius” (
12–
14). However, our results show no evidence for this. Risk for schizophrenia in our highest IQ category (mean IQ, 129; 0.19% of the sample) was lower than that in the next highest group (mean IQ, 122; 0.24% of the sample).
Third, we sought to clarify the nature of the association between IQ and schizophrenia. Both intelligence and schizophrenia risk are strongly familial, largely because of genetic factors (
15–
17). Therefore, the IQ-schizophrenia association could plausibly arise because of genetic and/or familial-environment factors predisposing both to low IQ and risk for schizophrenia. This hypothesis is consistent with evidence that 1) several candidate genes for schizophrenia are associated with lower IQ (
18,
19); 2) identified copy number variants increase risk both for schizophrenia and for intellectual disability (
20); 3) an exome study in schizophrenia found enrichment in intellectual disability for loss-of-function de novo mutations (
21); 4) children at high genetic risk for schizophrenia were shown to have lower IQ (
22); and 5) one previous twin study demonstrated a strong negative genetic correlation between IQ and schizophrenia liability (
23).
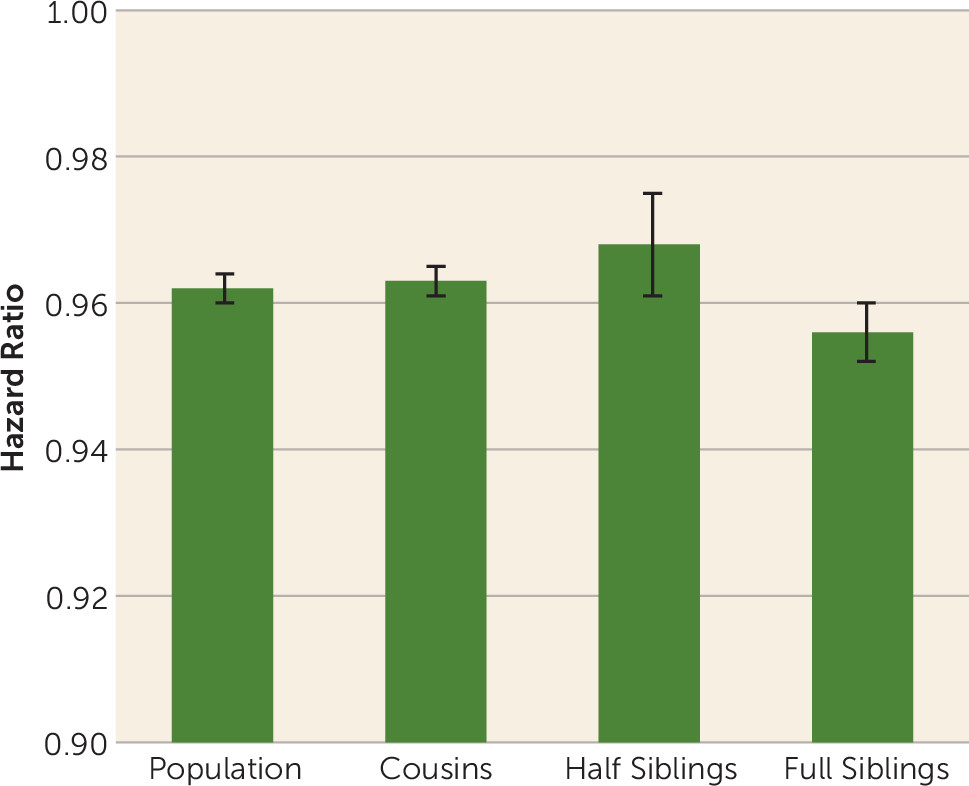
Our results were inconsistent with this hypothesis. Within pairs of relatives with differing IQs, the association between intelligence and schizophrenia was as strong as in the general population. Furthermore, no trend was seen for the association to be attenuated (that is, for the hazard ratio to approach unity) in siblings who share half their genes and are typically reared together compared with that seen in cousin pairs (who share one-eighth of their genes and are rarely reared together), or compared with that seen in the general population with no control for familial influences. However, our results are broadly congruent with a previous Swedish twin-sibling analysis that reports a very modest negative genetic correlation between IQ, as assessed at conscription, and schizophrenia (
24). The results of our co-relative analyses are most consistent with the hypothesis that some environmental risk factors not shared with close relatives have an impact on brain functioning in a way that both lowers IQ and predisposes to risk for schizophrenia.
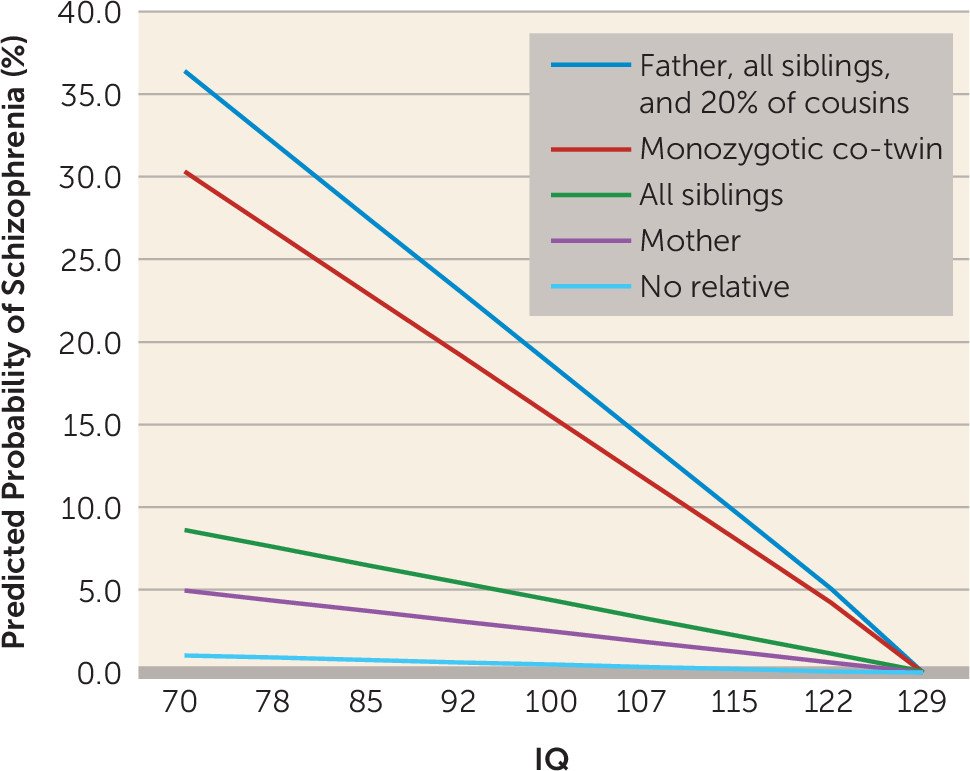
Our final goal was to illuminate the nature of the joint effects of genetic liability to schizophrenia and IQ on the risk for schizophrenia. We were particularly interested in distinguishing between additive effects—where liability to schizophrenia would reflect the independent contributions of genetic risk and IQ—and an interactive model in which the impact of genetic liability to schizophrenia is moderated by IQ. If we found an interactive model, would sensitivity to genetic effects be greatest in those with low or with high IQ?
Our results robustly supported an interactive rather than the additive model for the joint effects of genetic liability and IQ on risk for schizophrenia. Furthermore, the interaction arose because individuals with low IQ were much more sensitive to the effect of genetic liability than those with high IQ. That is, high IQ substantially attenuated the impact of genetic liability on risk for schizophrenia. We extended these findings by examining a broader definition of genetic liability as assessed in relatives (nonaffective psychosis), which produced similar findings. Follow-up analyses demonstrated that, consistent with our co-relative findings, the IQ-genetic liability interaction arose largely from IQ differences between close relatives.
This is not the place for speculative theories about why the still poorly understood genetic pathways of risk for schizophrenia might be more penetrant in individuals with low intelligence than in those with high intelligence. If our results are replicated, we can provide one important clue for neuroscientists and molecular geneticists. The changes in brain function that are expressed as low or high intelligence, and that convey sensitivity or resistance to the pathogenic effects of genetic liability to schizophrenia, appear to arise environmentally and will be seen most clearly in close relatives who differ in intelligence.
We are not the first to examine the relationship between IQ assessed in Swedish conscripts and subsequent risk for schizophrenia. Gunnell et al. (
25) and Zammit et al. (
26) examined 109,643 and 50,087 conscripts evaluated in the periods 1990–1997 and 1969–1970, respectively. As expected, our results on the association of IQ and schizophrenia are congruent with their findings, both of which were included in the recent meta-analysis (
3). The agreement between our results and those of the meta-analysis are thus slightly biased because of sample sharing, although only 9.6% of schizophrenia cases from the meta-analysis came from these two Swedish studies (
3).
Limitations
These results should be interpreted in the context of five potentially important methodological limitations. First, our sample was restricted to Swedish males. Second, we required a single hospital diagnosis of schizophrenia. Two studies found, using record reviews (
27) and diagnostic interviews (
28), that 96% and 94% of Swedish cases with hospital diagnoses of schizophrenia, respectively, met DSM-IV criteria for schizophrenia. When we narrowed our diagnosis to require at least two hospital diagnoses, the prevalence declined to 0.37%. Neither the linear effect of IQ on schizophrenia (hazard ratio=0.962, 95% CI=0.959–0.964) nor the results of the full-sibling co-relative control analyses (hazard ratio=0.957, 95% CI=0.952–0.962) changed appreciably. Third, IQ was available only as a global score in nine categories, the uppermost with a mean of 129. Therefore, we may have failed to detect small increases in risk for schizophrenia associated with very high IQ scores or with high levels of specific cognitive abilities. Fourth, using a planned risk-difference approach (
9), we observed a robust interaction in predicting schizophrenia from IQ and genetic liability. However, when these results were analyzed using a risk ratio approach, as implemented in Cox proportional hazards models, no significant interaction was found. To a first approximation, the impact of IQ and genetic liability to schizophrenia on risk for schizophrenia in this sample is the product of the two individual risks, not the sum. Fifth, diagnostic practices may differ by social class and, indirectly, by IQ. Such a bias is unlikely to explain much of the observed IQ-schizophrenia association because in this sample, IQ also strongly predicted the hazard ratio for other nonaffective psychoses (hazard ratio=0.971, 95% CI=0.969–0.972).