However, longitudinal data (
9–
17) on the association between CSVD and depressive symptoms are limited, and findings are mixed. Two previous studies (
12,
13) found an association between markers of CSVD and incident depressive symptoms. Additionally, three studies (
9,
15,
16) showed an association with increased severity or recurrence of depressive symptoms at follow-up but not with incident depressive symptoms, whereas two other studies (
11,
17) did not find any association with depressive symptoms. These mixed findings may be a result of study differences in sample size, follow-up duration, and the method of depression assessment (clinical interview [
12–
15] versus questionnaire [
9–
11,
16,
17]), as well as evaluation of different symptom clusters and differences in the source of populations investigated (selected [
9,
10,
12,
17] versus community-based samples [
11,
13–
16]). Furthermore, some studies did not adjust the results for potential important confounders, such as cognitive function (
11,
13,
14,
17) and cardiovascular factors (
12,
15,
17).
In view of the above issues, we investigated, in a large well-characterized cohort, the prospective association between, on the one hand, markers of CSVD (white matter hyperintensity volume, subcortical infarcts, cerebral microbleeds, Virchow-Robin spaces, and lower total brain parenchyma volume) and, on the other, incident depressive symptoms. We additionally investigated whether any such association was stronger for CSVD in brain regions involved in mood regulation (deep and frontal) compared with other regions (temporal and occipitoparietal).
Method
Participants
For the present study, we used longitudinal data from the Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study. The AGES-Reykjavik Study is a population-based cohort study originating from the Reykjavik Study, as described in detail elsewhere (
18). Briefly, from 2002 to 2006, a total of 5,764 surviving participants from the Reykjavik Study were examined. From 2007 to 2011, there was a follow-up examination of all surviving participants who agreed to participate (N=3,316). Reasons for not attending the follow-up examination included death (N=1,039), refusal (N=1,198), and lost to follow-up (could not be contacted by any means; N=211). The AGES-Reykjavik Study was approved by the National Bioethics Committee in Iceland (approval number, VSN-00–063) and by the National Institute on Aging Intramural Institutional Review Board. After complete description of the study was provided, written informed consent was obtained.
Depressive Symptoms
Depressive symptoms were assessed in all participants with the 15-item Geriatric Depression Scale (score range: 0–15) (
19,
20). Incident depressive symptoms were defined as a predefined Geriatric Depression Scale cut-off score ≥6 (
19,
20) at follow-up and/or new use of antidepressant medication (tricyclics, selective serotonin reuptake inhibitors [SSRIs], other nontricyclics, and monoamine oxidase inhibitors) assessed from medication bottles brought to the clinic. Individuals were excluded from the present analysis if they had depressive symptoms at baseline (score ≥6 and/or use of antidepressant medication at baseline).
Brain MRI Measures
Image acquisition.
All eligible participants were scanned with high-resolution brain MRI acquired on a study-dedicated 1.5-T system (Signa Twinspeed, General Electric Medical Systems, Fairfield, Conn.). The same imaging protocol was used in the 2002–2006 and 2007–2011 examinations, as described elsewhere (
21,
22), and included the following sequences: three-dimensional T
1-weighted spoiled-gradient recalled echo, proton density/T
2-weighted fast spin-echo, fluid-attenuated inversion recovery, and T
2*-weighted gradient-echo type echo planar. All images were acquired to give full brain coverage with slices angled parallel to the anterior commissure-posterior commissure line in order to yield reproducible image views in the oblique-axial plane.
Image analysis.
Several markers of CSVD were evaluated. White matter hyperintensity volume and total brain parenchyma volume (an indicator of cerebral atrophy) were computed automatically with a previously described image analysis pipeline (
23) and were expressed as the percentage of total intracranial volume. Lower total brain parenchyma volume was considered to be a marker of CSVD, since CSVD leads to generalized loss of brain parenchyma through, among other processes, microinfarcts (
24) and loss of white matter integrity (
25). Subcortical infarcts were evaluated as previously described (
21) and defined as brain parenchyma defects not extending into the cortex, with a minimum diameter of 4 mm and a signal intensity equal to CSF on all pulse sequences (T
2-weighted, proton density-weighted, and fluid-attenuated inversion recovery), and surrounded by an area of high-signal intensity on fluid-attenuated inversion recovery images. Parenchymal defects in the subcortical area with evidence of hemosiderin on the T
2*-weighted gradient-echo type echo planar scan were labeled as resorbed hematomas and were excluded from the definition of subcortical infarcts. Virchow-Robin spaces were evaluated separately and defined as defects in the subcortical area without a rim or area of high-signal intensity on fluid-attenuated inversion recovery and without evidence of hemosiderin on the T
2*-weighted gradient-echo type echo planar scan. Presence of Virchow-Robin spaces was considered to be a marker of CSVD, since they are associated with endothelial dysfunction, which may play a role in the pathogenesis of CSVD (
26). Cerebral microbleeds were defined as focal areas of signal void within the brain parenchyma visible on T
2*-weighted gradient-echo type echo planar scans and were identified as previously described (
22).
Potential Confounding Variables
As described elsewhere (
18), dementia case-finding was conducted at baseline and at follow-up according to a three-step procedure. Diagnosis of dementia (all subtypes) was made according to DSM-IV criteria by a panel that included a geriatrician, a neurologist, a neuropsychologist, and a neuroradiologist. The following variables were assessed by questionnaire: education (categorized as primary, secondary, and college/university education), smoking history (ever versus never), alcohol intake (high [>median] versus low [<median] consumers; median for women and men, 3.2 g per week and 8.0 g per week, respectively), and anxiety symptoms (presence versus absence). Presence of anxiety symptoms was defined by a positive response to any of the following questions: “In the past month, have you felt anxious or frightened?”; “Were there times lately that you felt anxious?”; “Are there special situations that make you anxious?”; and “Have you ever had attacks of fear or panic?” Presence of anxiety symptoms is a potential confounder because anxiety symptoms are frequently present in individuals with depression and are associated with cerebrovascular disease independently of depression (
27,
28). Gait speed, a measure of physical performance (
29), was the time needed to walk 6 meters at a usual pace. Hypertension was defined as systolic pressure >140 mmHg, diastolic pressure >90 mmHg, and/or use of antihypertensive medication. Body mass index (BMI) was calculated as measured weight divided by height squared. Diabetes was defined as a self-reported doctor’s diagnosis of diabetes, use of blood glucose-lowering medication, and/or a fasting blood glucose level ≥7.0 mmol/l. Coronary calcium score (categorized into sex-specific quartiles), a measure of atherosclerosis, was based on CT scanning. The Digit Symbol Substitution Test, a measure of psychomotor speech, and the Mini-Mental State Examination (MMSE), a measure of global cognitive function, were also administered (
18,
30).
Analytic Sample
Of the 3,316 participants who attended the follow-up examination, 709 had missing MRI data, and another 195 had missing data on depressive symptoms at baseline or follow-up. Missing MRI data was due to contraindications (N=278), refusal/nonattendance (N=360), or technical reasons (no qualitatively acceptable data available for all necessary sequences; N=71). In the remaining 2,412 participants, 138 were excluded because of a diagnosis of dementia at baseline (N=31) or follow-up (N=107). Finally, participants with presence of depressive symptoms at baseline (N=325) were excluded. Thus, the final study sample consisted of 1,949 participants. Participants excluded for the present analysis, compared with those who met inclusion criteria, were more likely to be older (mean age: 75.6 years compared with 74.6 years), female (60.8% compared with 56.6%), and less educated (primary school or less: 23.3% compared with 18.9%) and to have diabetes (12.4% compared with 9.1%), hypertension (82.0% compared with 76.9%), and/or stroke (9.1% compared with 5.2%) (all p values <0.05).
Statistical Analysis
The percentage of missing values with regard to potential confounders was minimal (maximum, 1.7%). We imputed these data using multiple imputation by chained equations (10 data sets) (
31). White matter hyperintensity volume was logarithmically transformed to normalize its skewed distribution.
The statistical analysis proceeded in several stages. Logistic regression analysis was used to estimate the association between markers of baseline CSVD (baseline white matter hyperintensity volume expressed per 1 higher standard deviation [per +1 SD], presence of any (coded 0, 1) subcortical infarcts, cerebral microbleeds and Virchow-Robin spaces, and total brain parenchyma volume expressed per 1 lower standard deviation [per –1 SD]) and incident depressive symptoms. Analyses were repeated looking prospectively at change in, or progression of, markers of CSVD as the determinant and development of depressive symptoms over the same period. These markers were as follows: an increase in white matter hyperintensity volume from baseline values (per +1 SD), any incident (coded 0, 1) subcortical infarcts, cerebral microbleeds and Virchow-Robin spaces, and a decrease in total brain parenchyma volume from baseline values (per –1 SD).
We repeated the above analyses for a priori selected brain regions, which included deep (subcortical) areas (the internal and external capsules, thalamus, striatum, hippocampus, and amygdala combined) and frontal, temporal, and occipitoparietal lobes. To efficiently summarize the pathology in each of these regions, we created a dichotomous composite score. This was calculated by assigning 1 point per CSVD marker based on the following cut-offs: regional white matter hyperintensity volume, quartile 4 versus quartiles 1–3; subcortical infarcts, cerebral microbleeds and Virchow-Robin spaces, ≥1 versus 0 lesion(s) per region; and regional brain parenchyma volume, quartile 1 versus quartiles 2–4. The points for each marker were combined to compute a dichotomous composite score per region, which indicated high (≥2 points) or low (0 or 1 point[s]) burden of CSVD. A separate composite score was computed for baseline CSVD and progression of CSVD over time, respectively.
Additionally, linear regression was used to evaluate the association between markers of CSVD and change in the Geriatric Depression Scale score over time as the outcome.
All models were adjusted for the following potential confounders: baseline age, sex, Digit Symbol Substitution Test score, MMSE score, education level, presence of anxiety symptoms, gait speed, alcohol use, smoking history, diabetes, BMI, hypertension, coronary calcium score, head coil, and follow-up time (model 1). Additionally, we carried out further adjustment for the baseline score on the Geriatric Depression Scale (model 2). The composite scores for each region were additionally mutually adjusted for each other, with the exception of the scores for the frontal and deep brain regions, which were not adjusted for each other because both regions are thought to be involved in mood regulation (
7,
8). We did not adjust the results for total disease load because total disease load includes the load per investigated region (as indicated by the composite scores) and, thus, can be considered an overadjustment (
32).
To test the robustness of the results, several sensitivity analyses were performed. Logistic regression analyses were repeated with incident depressive symptoms defined only as a score ≥6 on the Geriatric Depression Scale as the outcome, irrespective of new use of antidepressant medication. To minimize the potential confounding effect of stroke, analyses were repeated after excluding individuals with baseline stroke or incident stroke during follow-up. Additionally, to assess the possibility that depressive symptoms lead to CSVD (reverse “causality”), analyses were repeated with baseline presence of depressive symptoms as the exposure variable (individuals with baseline depressive symptoms were not excluded, N=325) and markers of progression of CSVD over time as the outcome. All analyses were conducted with PASW Statistics, version 21 (IBM, Armonk, N.Y.).
Results
The mean age of the study population at baseline was 74.6 years [SD=4.6], and 56.6% were women (
Table 1). In total, 10.1% (N=197) of the participants had incident depressive symptoms; of whom, 38.1% (N=75) had a Geriatric Depression Scale score ≥6, and 70.6% (N=139) had started using antidepressant medication (tricyclics, N=22; SSRIs, N=85; and other antidepressant medication, N=43). The mean time between the baseline and follow-up examination was 5.2 years (SD=0.2).
The results of the analyses with regard to markers of baseline CSVD showed that subcortical infarcts and lower total brain parenchyma volume had a statistically significant association with higher incidence of depressive symptoms, after adjustment for potential confounders (model 1 [see
Table 2]). Further adjustment for baseline Geriatric Depression Scale scores did not materially change these results (model 2).
Additionally, results of the analyses with regard to markers of progression of CSVD over time showed that an increase in white matter hyperintensity volume over time, incident subcortical infarcts, and Virchow-Robin spaces and a decrease in total brain parenchyma volume over time had a statistically significant association with higher incidence of depressive symptoms (models 1 and 2 [see
Table 2]).
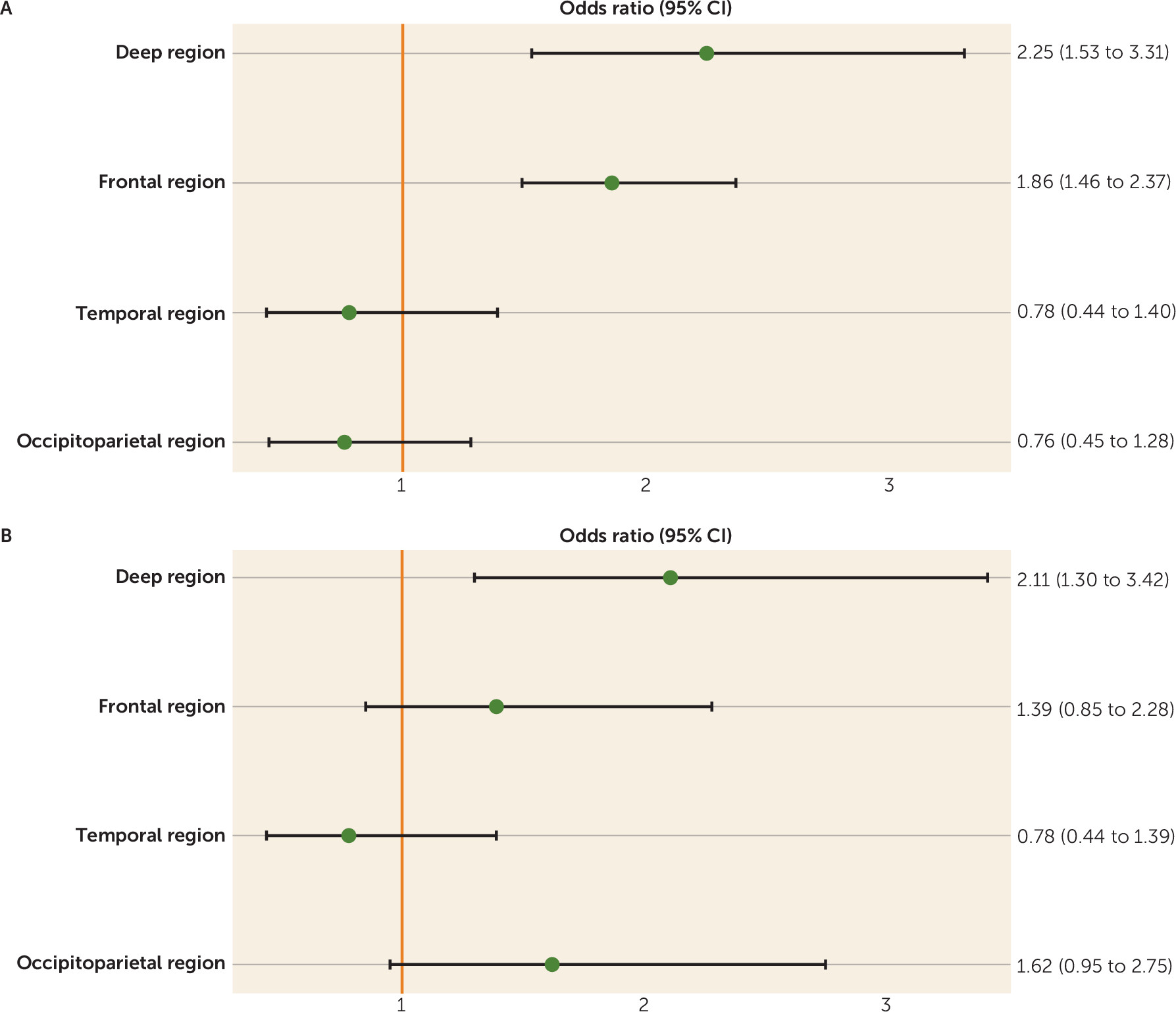
The composite scores at baseline (
Figure 1A) and the change in pathology over time (
Figure 1B) in the deep brain region were most strongly associated with higher incidence of depressive symptoms and reached statistical significance. Furthermore, the composite baseline score of the frontal brain region showed a statistically significant association with higher incidence of depressive symptoms.
The results of the analyses with change in Geriatric Depression Scale score over time as the outcome were qualitatively similar to the results of the analyses with incident depressive symptoms (models 1 and 2 [see
Table 3]). The association for a greater Geriatric Depression Scale score over time was statistically significant for higher baseline white matter hyperintensity volume, an increase in white matter hyperintensity volume over time, baseline and incident subcortical infarcts, incident cerebral microbleeds, and baseline and incident Virchow-Robin spaces and a decrease in total brain parenchyma volume over time (models 1 and 2 [see
Table 3]).
Sensitivity Analyses
Results were qualitatively similar when we repeated the analyses with incident depressive symptoms defined only as a Geriatric Depression Scale score ≥6, irrespective of new use of antidepressant medication (see Table S1 in the data supplement accompanying the online version of this article). When we excluded individuals with baseline stroke or incident stroke during follow-up (N=152), results did not materially change (see Table S2 in the online data supplement). Presence of depressive symptoms at baseline was not significantly associated with markers of progression of CSVD (see Table S3 in the data supplement).
Discussion
The present study investigated the association between markers of CSVD and incident depressive symptoms and had two main findings. First, various markers of CSVD were associated with higher incidence of depressive symptoms. This association was statistically significant for an increase in white matter hyperintensity volume over time, baseline and incident subcortical infarcts, incident Virchow-Robin spaces, lower total brain parenchyma volume at baseline, and a decrease in total brain parenchyma volume over time. These results were independent of cognitive function, education level, physical performance, anxiety symptoms, and cardiovascular factors. Additionally, results were qualitatively similar when change in the score on the Geriatric Depression Scale over time was used as the outcome instead of incident depressive symptoms. Second, CSVD located in the deep brain region, compared with other brain regions, was more strongly associated with higher incidence of depressive symptoms. To our knowledge, this is the clearest demonstration to date that CSVD is a risk factor for depressive symptoms.
Our findings are in accordance with previous cross-sectional studies (
13,
14,
33), which consistently have shown an association between markers of CSVD and depression. Additionally, two previous, smaller longitudinal studies, the 3-City-Dijon Study (
13) and the LADIS (Leukoaraiosis and Disability) Study (
12), found an association between higher white matter hyperintensity volume at baseline and higher incidence of depressive symptoms after a follow-up of 4 and 3 years, respectively. Furthermore, the SMART (Second Manifestations of ARTerial disease-Magnetic Resonance)-Medea Study (
9) found an association between lacunar infarcts in deep white matter tracts and an increased severity and more fluctuating course of depressive symptoms, in particular motivational/apathy related-symptoms of depression, during a follow-up of 3.5 years. The Cardiovascular Health Study (
16) and a neuroimaging substudy of the Rotterdam Study (
15) showed an association between higher white matter hyperintensity volume and/or cerebral infarcts, on the one hand, and worsening and recurrence of depressive symptoms, on the other, after 4 years of follow-up. In contrast, two other longitudinal studies, a post hoc analysis of a clinical trial (
17) and the Baltimore Longitudinal Study of Aging (
11), did not find any association between white matter hyperintensity volume at baseline and incident depressive symptoms. However, the latter studies were relatively small (N<550) (
11,
17) and/or had a relatively short follow-up duration (<3 years) (
17), which may have led to an underestimation of the association between CSVD and depressive symptoms. The present study extends previous research because of its large population-based sample of older individuals, long follow-up duration, the comprehensive brain MRI assessment of a wide spectrum of CSVD markers determined at baseline and at follow-up, and the extensive characterization of participants that enabled us to adjust for a series of potential confounders.
CSVD may lead to depressive symptoms through damage to deep and frontal brain structures involved in mood regulation (
7,
8). In accordance, the present study found that CSVD in the deep brain region, compared with other regions, was more strongly associated with higher incidence of depressive symptoms, although the 95% confidence intervals of the odds ratios for disease in the deep brain region did overlap with those of other regions.
However, other underlying mechanisms may explain the observed associations. First, it has been suggested that the association between CSVD and depressive symptoms exists because late-life depressive symptoms represent an early manifestation of (vascular) dementia (
34). For the present study, however, we excluded individuals with dementia at baseline or at follow-up. In addition, our results were adjusted for scores on the Digit Symbol Substitution Test and the MMSE, tests that evaluate multiple cognitive functions (
35). Second, other factors may be independently related to both CSVD and depressive symptoms, such as anxiety, cardiovascular factors, and stroke. However, the associations between CSVD and incident depressive symptoms were independent of anxiety symptoms and cardiovascular factors. Furthermore, the associations did not materially change when we excluded individuals with stroke. Third, we cannot exclude the possibility that the observed associations reflect reverse causation. Indeed, previous studies (
36,
37) have shown an association between depression and incident cardiovascular disease, including cerebrovascular disease. Although it is not fully understood how depression might lead to vascular disease, possible mechanisms include low-grade inflammation, endothelial dysfunction, platelet dysfunction, and unfavorable lifestyle habits (
38). It is unclear, however, why depression would lead to vascular disease in specific brain regions (e.g., the deep brain region involved in mood regulation). Additionally, in the present study, presence of depressive symptoms at baseline was not found to have a statistically significant association with markers of progression of CSVD over time, although the 95% confidence intervals of the effect estimates do not exclude the possibility of such an association. Fourth, it has been suggested that associations between CSVD and depression may be (partially) attributable to apathy (
39). Apathy overlaps with depression but may be a distinct syndrome (
40). In the present study, we did not evaluate apathy, and this issue requires further study.
Our study showed that most markers of progression of CSVD over time were associated with incident depressive symptoms but only some markers of baseline CSVD. This may be due to the design of our study with exclusion of individuals with depressive symptoms at baseline. This may have led to an underestimation of the association between baseline CSVD and development of depressive symptoms but not between progression of CSVD over time and depressive symptoms because individuals with depressive symptoms at baseline were most likely those with the strongest association between lifetime accumulation of CSVD (which is reflected by baseline CSVD) and depressive symptoms.
We analyzed depressive symptoms as both a dichotomous and a continuous outcome. The results of these analyses were qualitatively similar, except that more associations were statistically significant with change in the continuous score on the Geriatric Depression Scale than with the dichotomous incident depressive symptoms variable. This difference may be due to the fact that, in general, analyses with a continuous outcome have higher statistical power than analyses with a dichotomous outcome. Indeed, studying depression on a continuum has the merit that not only information on extremes is used but that all available information is exploited.
There are a number of limitations to the present study. First, incident depressive symptoms were assessed by questionnaire and use of antidepressant medication but not by a structured interview. Therefore, no information was available on clinical depression. Nevertheless, the sensitivity and specificity of questionnaire measures compared with a depression diagnosis based on a structured interview are high (>80%) (
20). Yet, the prevalence of depressive symptoms is greater, in particular in older individuals (
2,
4). Furthermore, late-life depressive symptoms, even in the absence of a diagnosis of major depressive disorder, are associated with greatly increased morbidity and mortality risk (
5,
6). Second, misclassification of incident depressive symptoms may have occurred because antidepressant medication is also prescribed for other reasons. However, the results were qualitatively similar when Geriatric Depression Scale scores alone were used as the outcome. Third, the present study is the first, to our knowledge, to evaluate the association between brain region-specific composite scores and depressive symptoms, and further study is therefore needed to confirm the present findings (e.g., using voxel-based morphometric analysis). Fourth, a limitation of the analysis with markers of progression of CSVD over time as the determinant is that progression of CSVD and incident depressive symptoms occur in the same time interval and cannot be assigned to a given time point within this interval. Finally, we used cerebral atrophy and Virchow-Robin spaces as markers of CSVD. Cerebral atrophy is, however, an indirect measure of vascular disease and is also strongly determined by other factors, in particular the process of neurodegeneration. We therefore cannot exclude the possibility that the association between lower total brain parenchyma volume and depressive symptoms is a result of factors other than CSVD. Furthermore, the etiology of Virchow-Robin spaces is currently incompletely understood, and this issue requires further study.
In conclusion, the present study shows that most markers of progression of CSVD over time and only some markers of baseline CSVD are independently associated with a concurrent development of higher incident depressive symptoms. From a clinical point of view, this association is important because it suggests that CSVD is a target for treatment and prevention strategies of late-life depression. Further study is needed to elucidate which factors contribute to CSVD and whether such factors can be therapeutic targets for late-life depression.