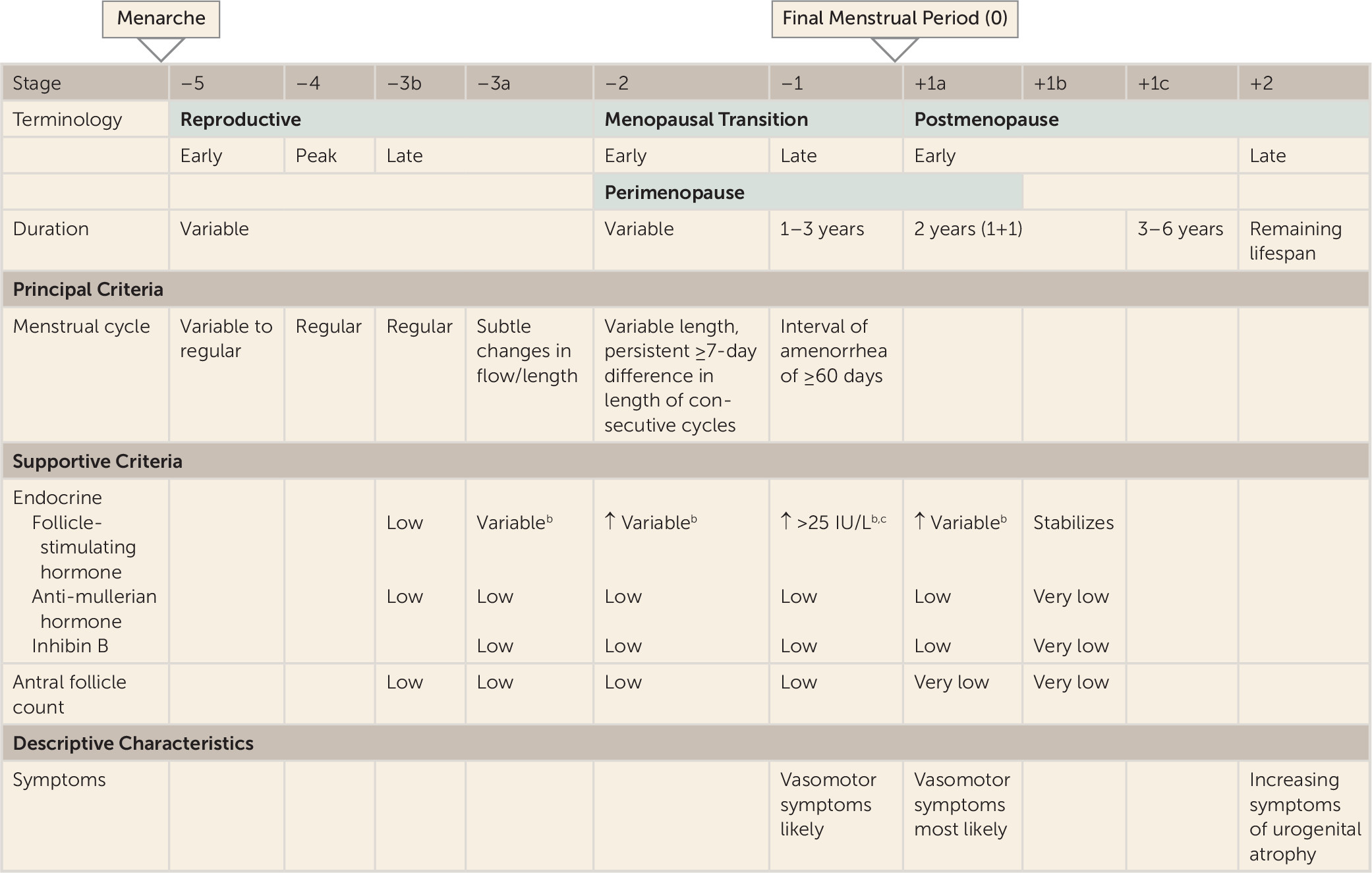
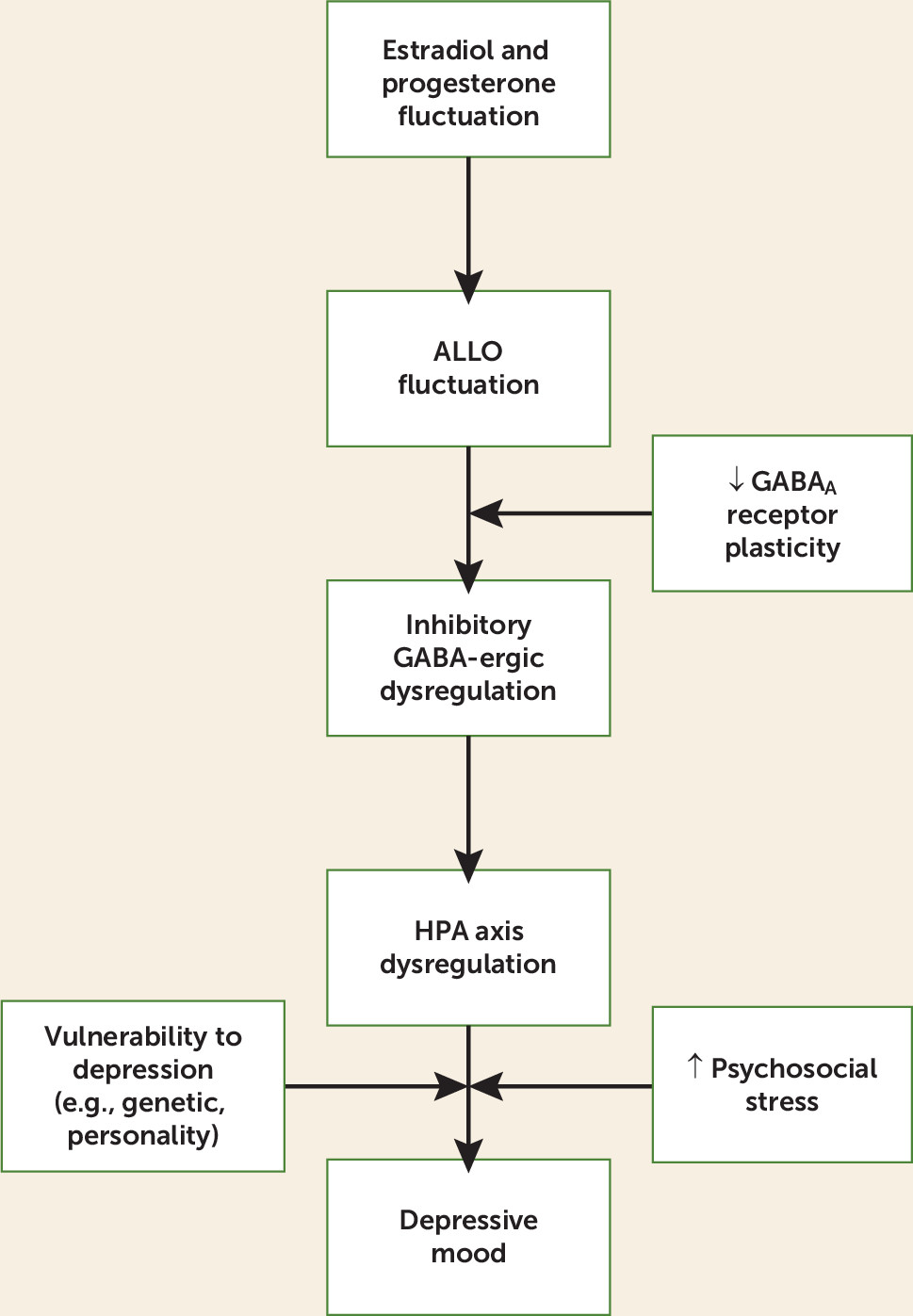
While the etiology of perimenopausal depression is not well understood, most studies suggest it is not simply due to low basal hormone concentrations. It has been hypothesized (for instance, in references
41 and
42) that the ovarian hormone
fluctuations that characterize the menopause transition trigger mood disturbances in vulnerable women. To our knowledge, five studies have evaluated the hormone variability hypothesis by examining naturally occurring fluctuations in ovarian hormones in relation to mood among women in the menopause transition (reviewed in reference
41). The first of these, the Massachusetts Women’s Health Study (
43) measured serum estradiol annually for 3 years in 309 women ranging from premenopausal to postmenopausal (STRAW stages –3 to +1) and found no association between estradiol variability and score on the Center for Epidemiologic Studies Depression Scale (CES-D). In a subset of participants in the Seattle Midlife Women’s Health Study, CES-D score was not associated with a urinary metabolite of estradiol, FSH, or testosterone in 131 women in STRAW stage –3 or –2 at baseline (
18). In that study, the CES-D was administered annually for 8 years and hormone concentrations were measured monthly for 4 years and then quarterly for 4 years. However, only 714 observations were collected in total, suggesting that, on average, participants provided five samples over the course of the 8-year study. Bromberger et al. (
22) also reported that among 3,302 SWAN participants, estradiol or FSH variability, calculated across eight annual measurements, was not associated with depressive symptoms. However, independent of menopausal status, testosterone levels and the change (increase) in testosterone from baseline were positively associated with CES-D score. While, overall, the above studies do not support a relationship between estradiol or FSH variability and mood, the absence of a positive finding may be related to the infrequent hormone sampling. Given the considerable hormonal variation occurring in the menopause transition, such infrequent measurements may be limited in their ability to capture the dynamics of the hormonal environment characterizing the menopause transition.
In contrast, Freeman et al. (
20) found that over 8 years in the Penn Ovarian Aging Study, clinical elevations in depressive symptoms and syndromal major depressive disorder were more likely to occur at times when estradiol variability was highest. Ten estradiol and FSH assessment periods occurred over the 8 years, each consisting of two blood draws taken 1 month apart. Estradiol variability at each assessment was calculated as the standard deviation across the two estradiol levels obtained during each assessment period. In cycling women, these measurements were taken in the early follicular phase. An important finding was that the relationship between estradiol variability and depressive symptoms continued to be significant after adjustment for increases in poor sleep, which may also accompany periods of increased hormonal flux. This study has several strengths that may explain its ability to detect a relationship between estradiol variability and perimenopausal depression. First, unlike the Massachusetts Women’s Health Study and the Seattle Midlife Women’s Health Study, the Penn study included only euthymic participants at baseline, ensuring that they were examining depression with onset in the menopause transition rather than major depressive disorder that began prior to, and continued into, the menopause transition. Second, the Penn study used more frequent hormonal assessments (twice annually, 1 month apart) than the aforementioned studies. A study by Daly et al. (
44), which also employed more frequent hormone sampling, assessed FSH weekly in 110 women with documented onset of depression in the menopause transition and found that the women who experienced a 50% drop in FSH over 6 weeks, indicating a return to premenopausal ovarian function, experienced a significant decline in depressive symptoms. Future research using more frequent assessments of depressed mood and ovarian hormone concentrations and isolating depression with onset during the menopause transition may therefore more definitively implicate the involvement of hormonal variability in the etiology of perimenopausal depression.
GABA-Ergic Neurosteroids in the Menopause Transition
While most studies have focused on the role of estradiol in the risk of perimenopausal depression, evidence from animal models suggests that ovarian hormone fluctuation increases the risk of perimenopausal depression, at least in part, because of the concurrent changes in progesterone-derived neurosteroids. Among the most studied progesterone-derived neurosteroids in humans is ALLO, an A-ring-reduced metabolite of progesterone. ALLO is stress responsive in animals and humans (for a review, see reference
47) and serves as a potent, positive allosteric modulator of GABA
A receptors through dose-dependent enhancement of GABA-induced chloride-ion channels (
48). GABA is the chief inhibitory neurotransmitter in the mammalian central nervous system. The role of GABA in regulating the HPA axis in response to stress by limiting the extent and duration of the HPA axis stress response is well established (
49). In part, it is through ALLO’s modulation of the GABA
A receptor to increase GABA-ergic transmission that ALLO not only negatively modulates the HPA axis to return it to homeostasis following stress (
50) but also exerts profound anxiolytic (
51) and antidepressant (
52) actions. However, ALLO’s anxiolytic properties may also be partially mediated through its effects on the bed nucleus of the stria terminalis, the “relay center” linking stress-responsive pathways such as the HPA axis and limbic structures such as the amygdala (
53). Two major sources of ALLO in women of reproductive age are the adrenal glands and the corpus luteum, where ALLO is converted from progesterone (
54). Because of ovarian ALLO contributions, ALLO concentrations in premenopausal women are highest in the luteal phase and lowest in the follicular phase (
54). However, in postmenopausal women, the adrenal glands become the exclusive source of peripheral ALLO (
54). It is important to note that peripherally derived ALLO freely crosses the blood-brain barrier (
55) and contributes significantly to CNS concentrations (
56).
As mentioned earlier, an increasing proportion of anovulatory cycles results in less frequent luteal phases and therefore overall lower levels of progesterone. Although the availability of progesterone is an important determinant of ALLO, estradiol is also likely to positively influence ALLO production through its modulation of the enzymes involved in the conversion of progesterone to ALLO, 5α-reductase and 3α-hydroxysteroid dehydrogenase (
57). This is supported by both basic and clinical research. For example, while ovariectomy in animals has been shown to decrease ALLO concentrations in the hippocampus, hypothalamus, pituitary, and plasma, transdermal estradiol administration restores preovariectomy brain and plasma ALLO concentrations (see reference
57 for a review). Similarly, transdermal estradiol increases plasma ALLO in postmenopausal women (
58,
59). Thus, even in the absence of ovulation and the consequent production of progesterone, intermittent endogenous production of estradiol during the menopause transition may cause fluctuations in the synthesis and release of ALLO.
ALLO fluctuation may have important implications at the GABA
A receptor, which is composed of a combination of five (out of 19 existing) subunits. Which subunits a receptor contains will greatly influence its sensitivity to neurosteroids (see reference
60 for a review). For example, the δ subunit has been shown to greatly increase receptor sensitivity to very low concentrations of neurosteroids; mice lacking the δ subunit therefore exhibit greatly reduced neurosteroid sensitivity (
61). In contrast, GABA
A receptors containing the ε subunit are relatively insensitive to neurosteroids like ALLO. Furthermore, the subunit composition of GABA
A receptors is extremely plastic and influenced by neurosteroid levels; for example, during pregnancy, when levels of progesterone, and thus progesterone-derived neurosteroids, are extremely elevated, the expression of δ subunits is down-regulated in multiple areas of the brain, thus reducing receptor sensitivity to elevated ALLO levels (
62).
This ability of the GABA
A receptor to change its composition in response to ALLO concentrations is likely to be especially important in times of considerable ALLO flux. Failure of the GABA
A receptor to match its composition to an ever-changing hormonal environment could result in either too high or too low GABA-ergic inhibitory tone. In addition, ovarian hormone fluctuation may actually trigger maladaptive changes in GABA
A receptor configuration. Evidence supporting this comes from animal models of puberty, in which ovarian hormone fluctuations have been shown to promote the expression of GABA
A receptors containing α4, β2, and δ subunits in rodents, the combination of which has been found to transform ALLO’s effects from excitatory to inhibitory at the GABA
A receptor (
63). Thus, rather than positively modulating the GABA
A receptor, ALLO inhibits it. In turn, there is an overall decline in GABA-ergic inhibitory tone. Furthermore, this reduction in GABA-ergic tone during puberty is also accompanied by an increase in anxiety, as indicated by less time spent in the open arms of the elevated plus maze and more anxiety behavior following a restraint stress (
63). Hormone fluctuation across the estrous cycle (
64) or progesterone (and therefore ALLO) withdrawal induced in the laboratory (
65) have also been shown to result in similar changes in GABA
A receptor subunit expression.
Failure of the GABA
A receptor to demonstrate adaptive homeostatic plasticity in the context of steroid hormone fluctuations is theorized to be involved in the development of PMDD and postpartum depression (see references
60,
62, and
66 for reviews). This may be one mechanism contributing to decreased saccadic eye velocity, an indirect measure of GABA
A receptor sensitivity, in women with PMDD during their symptomatic phase (
67). In light of this, we propose that insufficient plasticity of the GABA
A receptor in the menopause transition, or maladaptive changes to the GABA
A receptor, may contribute to mood disturbance during the menopause transition, when concentrations of both estradiol and progesterone become erratic and unpredictable. How GABA
A receptors “respond” to fluctuating ALLO concentrations in the menopause transition would be critical in determining overall GABA-ergic tone, mood, and, theoretically, regulation of the HPA axis. This process, in which hormonal fluctuation can trigger GABA
A subunit changes such that ALLO’s effects become paradoxical (inhibitory rather than excitatory at the GABA
A receptor; anxiogenic rather than anxiolytic), may shed light on the results of a study by Andréen et al. (
68). This study of 36 women in late perimenopause or early postmenopause who were treated with progesterone supplementation showed that women with resultant medium ALLO concentrations reported significantly more negative mood when compared with women with low ALLO levels. The possibility exists that the context of the perimenopausal fluctuating hormonal environment to which these women were exposed had contributed to GABA
A receptor subunit changes with consequent alterations in ALLO’s effects at the receptor, and in turn, its effects on mood (
69).
To the extent that GABA-ergic dysregulation is involved in perimenopausal depression, genes coding for GABA
A receptor subunits may be implicated in predisposing some individuals to respond maladaptively to ALLO fluctuations and thus be at increased risk for perimenopausal depression. GABA
A receptor subunit gene polymorphisms are differentially associated with risk for other mental disorders, such as alcohol dependence (
70), major depressive disorder (
71), bipolar disorder (
71), and schizophrenia (
71). In animal models, mice genetically designed to lack the δ subunit do not exhibit ALLO’s paradoxical effects at the GABA
A receptor during puberty that are seen in wild-type mice (
63). An investigation of which GABA
A receptor subunit gene polymorphisms are associated with an increased risk for perimenopausal depression may be warranted. Large-scale epidemiologic studies such as SWAN may provide appropriate specimens for such genotyping.
Although ALLO’s effects on the GABA-ergic system are the best characterized and are thus the primary focus of this review, alterations in overall GABA-ergic tone are likely to impact the release of other neurotransmitters relevant to the development of psychopathology. For example, recent in vitro studies of rodent hippocampal neurons suggest that ALLO, via presynaptic GABA
A receptors, modulates glutamate release (
72). ALLO’s effects on glutamate may be particularly relevant to the study of perimenopausal depression in light of the recent recognition that glutamate transmission is likely a key player in the etiology of major depressive disorder and other psychopathologies (
73).
HPA Axis Dysregulation in the Menopause Transition
GABA plays a critical role in regulating the HPA axis and limiting HPA activation following exposure to stress (
49). As such, any failure of the GABA
A receptor to adapt appropriately to the hormonal environment can be expected to have direct consequences for HPA axis activity. For example, if ALLO were to negatively modulate the GABA
A receptor in response to hormonal fluctuation (as opposed to serving as a positive allosteric modulator of the GABA
A receptor), as is seen in animal models of puberty, this would contribute to an overall increase in HPA axis activity since the GABA-ergic inhibition of the HPA axis is removed. Under these conditions, because the ALLO concentration increases following stress, this could potentiate HPA axis reactivity and prolong recovery in response to stress. In this way, we speculate that menopause-transition-related ovarian hormone fluctuation could trigger HPA axis dysregulation.
Dysregulation of the HPA axis in major depression has frequently been described as one of the most consistent findings in psychiatry (
74). However, only in the last decade have we begun to view altered HPA axis activity as a risk factor increasing one’s susceptibility to depression rather than a consequence or epiphenomenon of depression (
75). This view is supported not only by prospective studies identifying an elevated cortisol level as a precursor to the onset of first-episode major depression (
76), as well as relapse (
77), but also by studies observing increased HPA axis activation among the euthymic relatives of individuals with a history of depression, compared with control subjects with no family history of depression (
78). Research on postpartum depression (
79,
80) and PMDD (see reference
81 for a review) also suggest that HPA axis dysregulation has pathophysiological relevance to reproductive mood disorders.
To date, little is known about HPA axis activity in the menopause transition. Komesaroff et al. (
82) examined cortisol reactivity to the Trier Social Stress Test, a speech and arithmetic stressor battery, following 8 weeks of oral estradiol or placebo in women during the menopause transition. It was found that estradiol therapy resulted in an attenuated cortisol response to the stressor. Two additional studies have examined basal cortisol levels in the menopause transition. In the Seattle Midlife Women’s Health Study, 91 women provided monthly first-morning urine specimens for cortisol measurement as they transitioned across menopausal stages (early to middle, middle to late, and late menopause transition to postmenopause) (
83). It was found that 68% of the 22 women transitioning from the middle to late menopause transition exhibited an increase in cortisol, the magnitude of which has previously been associated with a decrement in memory performance in older women (
84) and may therefore have clinical significance. While the increase in cortisol during the late menopause transition was not associated with depressive symptoms in this community sample, this study was limited in that it examined basal cortisol and not cortisol reactivity to stress. A study by Schmidt et al. (
85) found no difference in the basal cortisol levels of 24 women with perimenopausal depression when compared with 26 asymptomatic control subjects. However, again, this study did not examine cortisol reactivity; it also did not account for STRAW stage. Together, these studies suggest that perimenopausal depression may not be associated with alterations in
basal HPA axis hormone concentrations but that ovarian hormones do regulate HPA axis responses to stress in women during the menopause transition. To our knowledge, there have been no studies examining HPA axis activation in response to stress in women with perimenopausal depression.