Chronic psychotic disorders are estimated to occur in more than 3% of the population, and they contribute to a considerable amount of morbidity worldwide (
1). According to the Global Burden of Diseases, Injuries, and Risk Factors Study, two of these illnesses, schizophrenia and bipolar disorder, accounted for over 27 million years lived with disability in 2010 (
2). Schizophrenia has been shown to reduce the lifespan of sufferers by more than 18 years because of factors such as substance use, poverty, neglect of personal well-being, smoking, metabolic syndrome, and suicide (
3,
4).
Antipsychotic drugs are the mainstay of treatment for psychosis, yet they are associated with substantial heterogeneity in their therapeutic efficacy (
5,
6). Nonresponse to standard antipsychotic agents contributes to poor functional outcomes and a large economic impact on health care systems, including up to a tenfold increase in total health resource utilization (
7,
8). Treatment algorithms for these illnesses are devoid of prognostic measures, and clinicians often resort to trial and error when faced with the potential inefficacy of their treatment choices. Moreover, when patients do not have an adequate clinical response to treatment, they may endure prolonged periods of untreated illness. There is some evidence that nonresponse within the first 2 weeks of treatment may ultimately predict a failed treatment trial, yet even this approach requires costly trial and error (
9,
10). Moreover, this finding is limited to patients with chronic illness; for patients with first-episode schizophrenia, it is recommended that antipsychotic drug trials last up to 16 weeks (
11).
Current practice suggests a need for reliable, biologically based prognostic measures of treatment response to antipsychotic agents. In the domain of neuroimaging, structural methods have shown that differences in cortical thickness, asymmetry, and gyrification may be associated with subsequent response to antipsychotic treatment (
12,
13). Reduced white matter integrity has also been linked to nonresponse to treatment in patients with first-episode psychosis (
14). One recent study demonstrated that functional connectivity of the ventral tegmental area is associated with treatment response (
15). Although important in explicating pathophysiological mechanisms, these findings have not been replicated in independent cohorts and have not resulted in predictive tests with clinical utility.
Multiple lines of evidence suggest that variation in the physiology of the striatum may be critical to antipsychotic treatment outcomes. This subcortical region contains a dense concentration of dopamine D
2 receptors, the shared target of all known antipsychotic agents. While genetic variation at the dopamine D
2 receptor has replicably been shown to influence response to these medications, the modest size of this effect limits its clinical utility (
16). Treatment-resistant schizophrenia has been associated with normal dopaminergic synthesis capacity in the striatum, while treatment responders demonstrate elevated striatal dopamine in psychotic illness (
17,
18). In congruence with cross-sectional data that has linked abnormal corticostriatal interactions with psychotic illness (
19,
20), we recently reported that improvement of psychotic symptoms is associated with changes in striatal functional connectivity over the course of treatment (
21). In light of these findings, we hypothesized that alterations in functional connectivity of the striatum may provide prognostic value in the treatment of psychosis.
Our aim in this study was to develop and test a prognostic biomarker, based on functional connectivity of the striatum, with the potential for clinical utility in the prediction of positive symptom response to antipsychotic treatment. We identified the putative biomarker in a discovery cohort of patients with first-episode schizophrenia, and then tested the results in a generalizability cohort of patients with chronic psychotic illness who were newly hospitalized for an acute psychotic episode.
Method
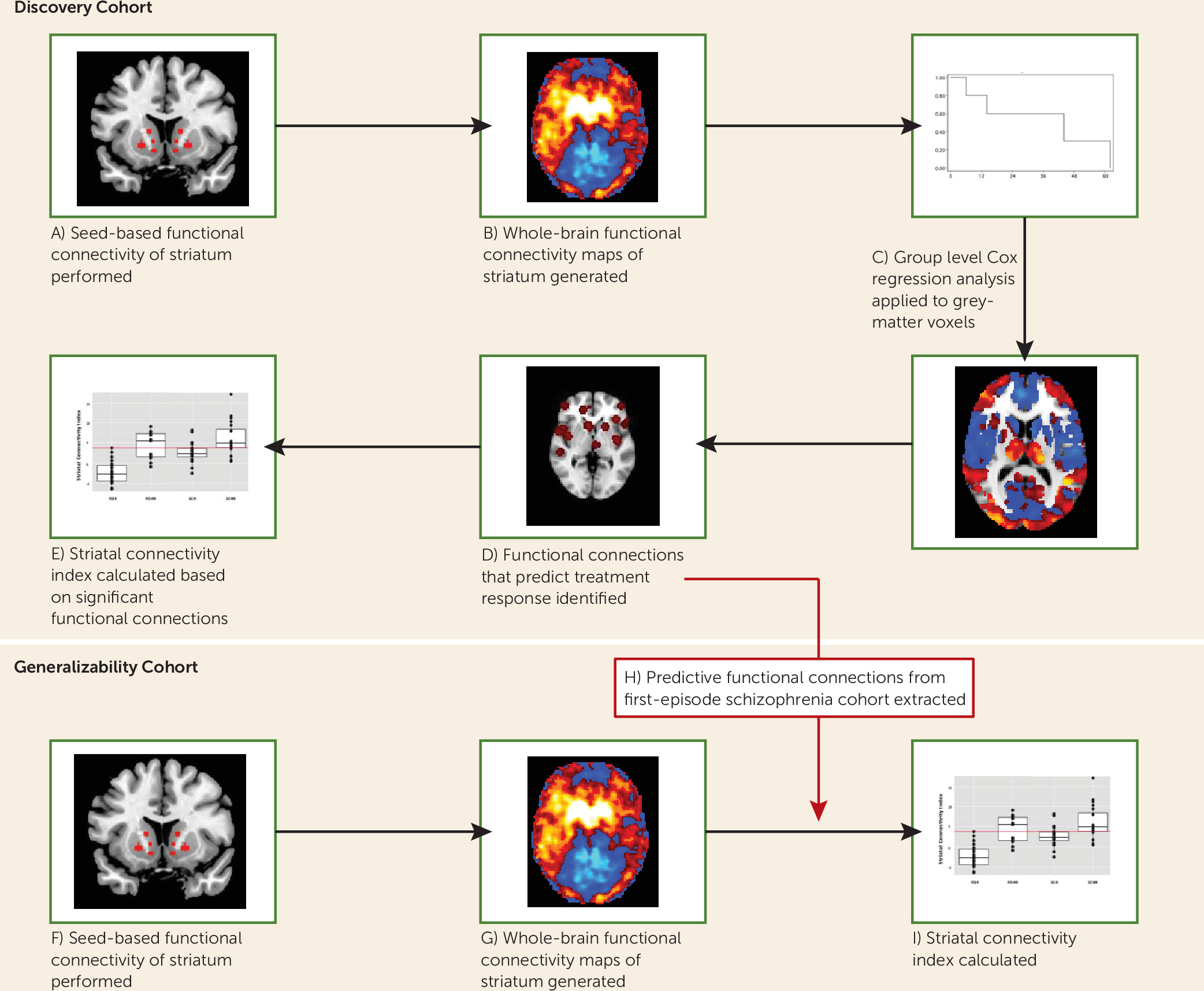
We developed and tested the putative biomarker in a stepwise manner, which we outline here, with further details provided in the sections below. As shown in
Figure 1, we identified targets of striatal functional connectivity that predicted response in a discovery cohort of patients undergoing controlled treatment for their first episode of schizophrenia. First, we placed seeds in predefined regions of interest within the striatum. Next, we obtained whole-brain resting-state correlation maps of the time series derived from these regions of interest. Striatal connectivity values from each voxel were then entered into Cox regression analyses to predict treatment response in the discovery cohort. Voxels that were significant in the Cox regression were then identified, and their striatal connectivity values were summed and weighted based on an independent cohort of healthy volunteers, in order to reduce overfitting. This weighted sum is a scalar value called the striatal connectivity index, and the constituent connections of interest identified from our discovery cohort can then be directly applied to any subsequent scans. Thus, in the present study, the striatal connectivity index was obtained in the generalizability cohort, by placing striatal seeds and obtaining their resting-state correlation maps. The key regions that predicted response in the discovery cohort were then directly selected on the generalizability cohort patients’ scans, and the connectivity values of these regions were weighted (based on the same cohort of healthy volunteers mentioned above) and summed to form the striatal connectivity index for each participant in the generalizability cohort. These striatal connectivity index values were then compared with observed treatment response and hospital length of stay for the patients in the generalizability cohort.
Participants
The discovery cohort consisted of 41 patients between the ages of 15 and 40 who were experiencing their first episode of a schizophrenia spectrum disorder (schizophrenia, schizophreniform disorder, schizoaffective disorder, or psychotic disorder not otherwise specified) and had no more than 2 weeks of cumulative lifetime exposure to antipsychotic medication. Patients underwent resting-state functional MRI (fMRI) scanning and symptom ratings before being assigned to double-blind randomized controlled treatment with either risperidone or aripiprazole for 52 weeks (see NCT00320671 at ClinicalTrials.gov). This analysis includes data only from the first 12 weeks of treatment, the acute treatment trial. Assessments with the Clinical Global Impressions Scale (CGI) and the Brief Psychiatric Rating Scale–Anchored Version (BPRS-A) were performed weekly during the first 4 weeks, then biweekly for the remaining 8 weeks of the acute treatment trial. Based on our previous work in first-episode patients (
22), treatment response was defined a priori as two consecutive visits with a CGI improvement score of 1 or 2 (much or very much improved) and a rating of 3 (mild) or less on all of the following items of the BPRS-A: conceptual disorganization, grandiosity, hallucinatory behavior, and unusual thought content.
The generalizability cohort consisted of 40 patients hospitalized at the Zucker Hillside Hospital for acute psychotic symptoms. Patients had diagnoses of schizophrenia, schizophreniform disorder, schizoaffective disorder, psychotic disorder not otherwise specified, or bipolar I disorder with psychotic features. The clinical management of these patients followed routine clinical guidelines and was not influenced by our research protocol but in all cases included antipsychotic medication. Patients were approached for participation in the study shortly after being admitted to the hospital. They underwent resting-state fMRI scanning and evaluation of symptoms with the BPRS-A at baseline and weekly during hospitalization until discharge or treatment response. Treatment response in this group was defined in the same way as it was in our first-episode schizophrenia group. However, absolute response criteria differed between our first-episode schizophrenia and generalizability cohorts because of the nature of the study designs. Patients in the former were evaluated in the context of a large clinical trial that allowed for frequent clinical ratings, while in the latter, patients were evaluated only during their hospitalization, the duration of which was determined by their clinicians and was usually less than 4 weeks. Our primary measure of treatment response in the generalizability cohort, a 20% reduction in severity ratings on core psychotic symptoms, was required to occur within the first 2 weeks of treatment, based on evidence from previous research that nonresponse within the first 2 weeks of treatment may ultimately predict a failed treatment trial (
9,
10). Length of hospital stay was used as a secondary measure of response.
The healthy volunteer sample consisted of 41 participants, with an age and gender distribution nearly identical to that of our first-episode schizophrenia cohort. It is important to emphasize that our hypotheses did not rely on direct comparison between first-episode schizophrenia patients and healthy volunteers. We used this healthy volunteer group for a more unbiased construction of weightings for our striatal connectivity index calculation, in order to minimize overfitting in the discovery cohort.
All participants received a complete description of the study and provided written informed consent according to a protocol that was approved by the Institutional Review Board of the North Shore-Long Island Jewish Health System. Additional methodological details have been published elsewhere (
21) and are included in the
data supplement that accompanies the online edition of this article.
Resting-State fMRI Image Acquisition and Preprocessing
Resting-state fMRI scans were collected on a GE 3-T scanner. Five-minute resting-state functional scans (150 whole-brain volumes) were acquired for each study participant. During the scan, participants were asked to close their eyes and were instructed not to think of anything in particular. The acquisition parameters and preprocessing methods are described in the
data supplement; notably, strict attention was paid to potential motion artifacts according to the methods described by Power et al. (
23).
Functional Connectivity Analyses
As mentioned earlier, our aim was to develop a potential biomarker of treatment response based on intrinsic functional connectivity between striatal subregions and the rest of the brain. To generate this index, a seed-based functional connectivity approach was applied to the striatum based on the methods of Di Martino et al. (
24), which we previously adopted (
21). Using these methods, we created 3.5-mm spherical regions of interest, bilaterally, in the dorsal caudate, ventral caudate, nucleus accumbens, dorsal rostral putamen, dorsal caudal putamen, and ventral rostral putamen (see Table S1 and Figure S1 in the online
data supplement). Once regions of interest were defined, the mean time course of resting-state blood-oxygen-level-dependent activity was extracted from each seed region. Whole-brain voxel-wise correlation maps for each region of interest were then created with the extracted waveform as a reference. The Fisher z-transformation was applied to the resulting correlation maps. Striatal connectivity maps for each striatal region of interest showed good correspondence with previous studies that utilized this method (
21,
24).
Voxel-Wise Survival Analysis
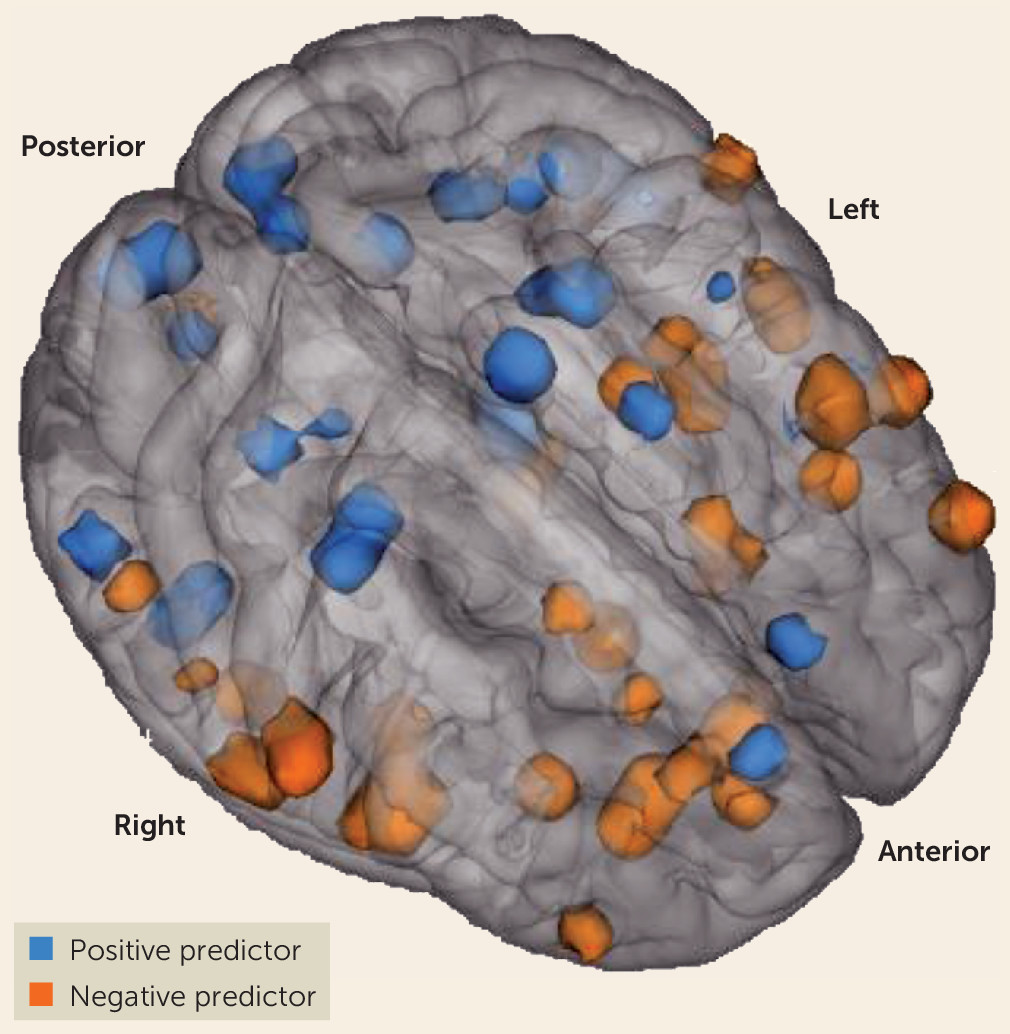
These striatal connectivity z maps were then utilized to develop the putative biomarker as follows: 1) for every voxel located within gray matter (181,144 voxels total), the corresponding connectivity strength for each first-episode patient was entered into a univariate Cox regression analysis along with clinical outcome (response or nonresponse) and time to outcome or study dropout (in weeks); 2) the resulting z scores of this analysis for each voxel were placed in Montreal Neurological Institute standard brain space to create whole-brain maps; and 3) at the group level, we performed one-sample t tests on these maps for each region of interest. While it is common in case-control studies of psychiatric disorders to apply Bonferroni-style corrections to functional neuroimaging data, the aims of our study dictated a different statistical approach. As our goal was to capture the maximal amount of treatment-related variance for our potential biomarker, we applied a threshold of p<0.005 to our analysis of the discovery data set. Once the significant elements (connections of interest) were identified and a striatal connectivity index was developed from these elements, our use of a fully independent generalizability cohort allowed for an unbiased estimate of the prognostic value. In the discovery cohort, a total of 91 functional connections across the 12 input regions of interest predicted treatment response in either a positive or a negative direction (
Figure 2; see also Tables S2 and S3 in the online
data supplement).
Striatal Connectivity Index Calculation
Next, a striatal connectivity index was computed using data from the 91 predictive striatal functional connections. In order to reduce circularity and minimize overfitting in this computation, we normalized data from the discovery cohort of first-episode schizophrenia patients with data from healthy participants. To do this, we extracted the raw correlational values of all 91 predictive striatal functional connections from connectivity maps of all participants in our healthy volunteer group as well as in our first-episode schizophrenia cohort. The mean and standard deviation were calculated on correlational values at each of the 91 functional connections from the healthy volunteer group and used to z-transform the patient data. A principal-components analysis was performed on the extracted functional correlational values in the healthy volunteer data set. The first principal component was computed. Loadings onto this first principal component across all 91 functional connections were applied to connectivity values in the first-episode cohort. Similarly, loadings onto that first principal component in the healthy volunteers were also applied to the raw connectivity values in the generalizability cohort. The sum of the products of all correlational values and loadings onto the first principal component was calculated for each patient. We called this value a striatal connectivity index, which represents the expression of the first principal component of striatal connections of interest from the healthy volunteer cohort.
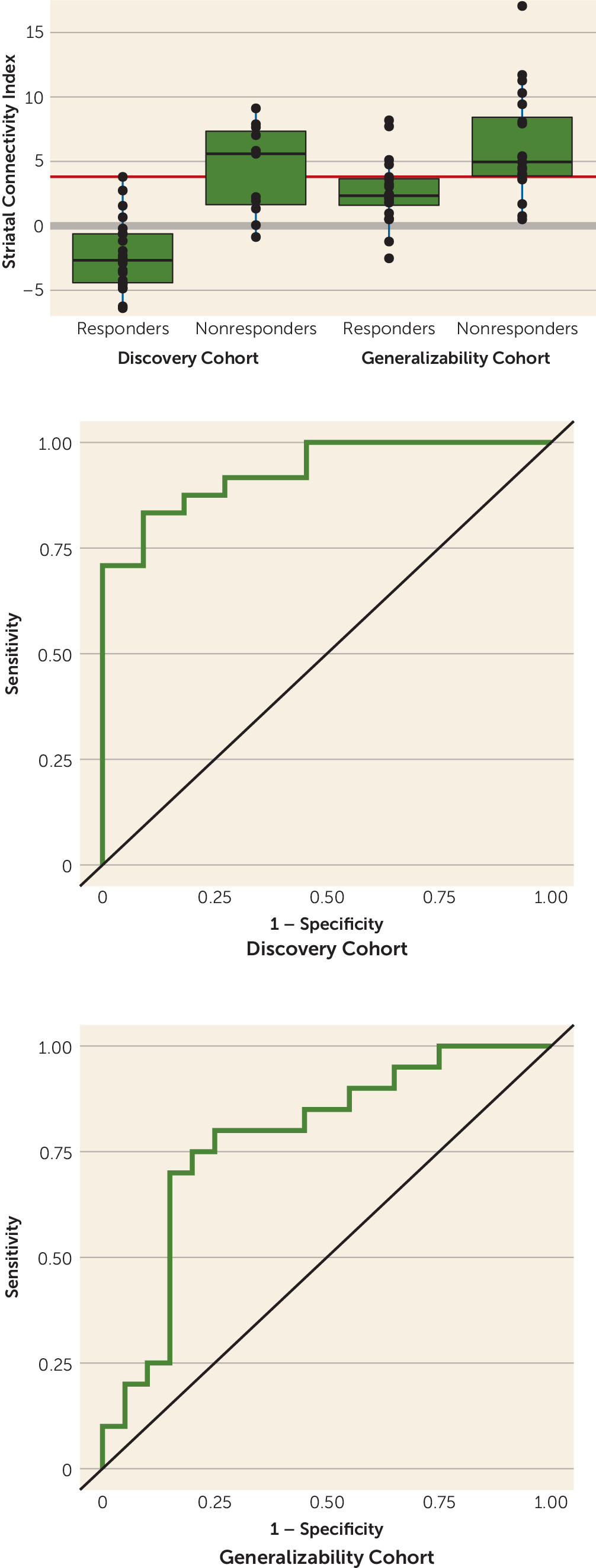
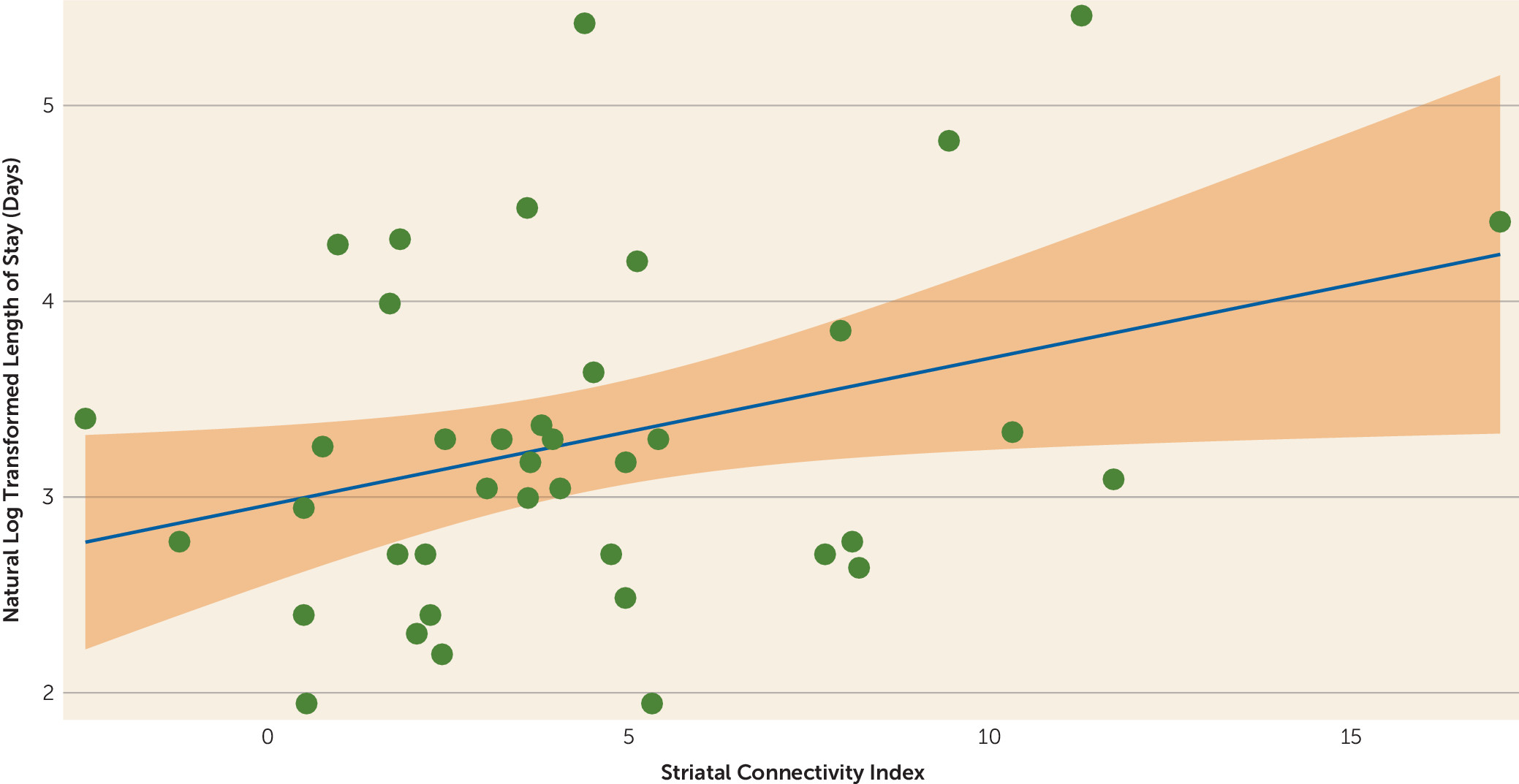
This index was examined for prognostic value against the predetermined response/nonresponse designation. A cutoff threshold for this striatal connectivity index was derived based on responder/nonresponder status in the discovery cohort, and the sensitivity and specificity of this threshold were tested in the generalizability cohort to determine clinical utility. The ability of the striatal connectivity index to predict length of stay was also examined in the generalizability cohort.
Discussion
In this study, we provide evidence for a potential baseline resting-state fMRI biomarker that predicts response to treatment with antipsychotic drugs in patients with psychotic disorders. We identified striatal functional connectivity nodes significantly associated with treatment response in a discovery cohort of patients with first-episode schizophrenia and developed a prognostic striatal connectivity index normalized using a group of demographically comparable healthy participants. We then applied this measure to a generalizability cohort of chronic patients undergoing treatment for acute psychotic symptoms. In both the discovery and generalizability data sets, we observed a significant separation between responders and nonresponders, with clinically meaningful levels of sensitivity and specificity. In addition, the prognostic index correlated with length of stay for psychotic symptoms in a large psychiatric treatment facility.
Our results are broadly consistent with other neuroimaging studies, across multiple modalities, demonstrating that antipsychotic drugs modulate functional activity and connectivity of the basal ganglia (
21,
26–
28). While there has been an abundance of neuroimaging research in psychotic disorders, there remains a crucial gap between this work and clinical practice. In particular, resting-state fMRI has provided insight into the intrinsic functional makeup of psychotic disorders but has yet to offer clinical utility. This method has been preliminarily examined as a prognostic measure in depression and chronic pain, as well as in schizophrenia (
15,
29,
30). Clinical assays derived from the method we describe have the potential to tease apart the clinical heterogeneity of psychotic disorders and to guide real-world treatment decisions.
Development of prognostic tools is required to bring contemporary “precision medicine” approaches to psychiatry. To our knowledge, this study is the first to report a prognostic fMRI-derived measure validated in an independent cohort of antipsychotic-treated patients. At present, however, the striatal connectivity index we describe here is insufficient to precisely target antipsychotic prescriptions to specific patients, for two reasons: first, all currently available antipsychotics are mechanistically and clinically similar (with the possible exception of clozapine), and second, the observed sensitivity (80%) and specificity (75%) of the striatal connectivity index suggest additional room for improvement.
With these limitations in mind, however, striatal connectivity index scores may be sufficiently accurate for more limited, but still clinically relevant, decision-making purposes. For example, a prognostic tool could be used in hospital settings to target limited clinical resources such as additional psychoeducation for patients and their families to reduce frustration with potentially longer treatment trials; nonadherence to care has been shown to be associated with increased rates of relapse and worsened outcomes for patients, and lack of initial efficacy is a prominent cause of nonadherence (
25,
31). Moreover, patients who do not remit are more likely to exhibit violent behavior and to need emergent care (
32); thus, additional social work support could be deployed for those most likely to have difficulty achieving stable discharge criteria. Outcomes such as nonadherence, relapse, and violence carry the dual burdens of additional patient suffering and greater cost to health care delivery systems (
7,
8).
Biomarkers such as the striatal connectivity index may also assist in the development of novel treatments for patients in several ways. First, patients entering clinical trials could be stratified on the basis of the striatal connectivity index, and noninferiority trials could be targeted to those patients who are likely to respond to known antipsychotic agents. Conversely, clinical trials involving agents with novel mechanisms may seek to target patients who are likely to be nonresponders to conventional D
2 antagonists. Additionally, the circuitry identified in the present study, combined with our previous demonstration of striatal circuits that are directly changed over time with efficacious treatment (
21), can provide an important index of “target engagement” for the development of novel therapeutics (
33). Of note, one of the prognostic connections, between the right ventral rostral putamen and the anterior cingulate, was previously shown, in a smaller subset of our first-episode schizophrenia patients, to increase in strength as psychotic symptoms resolved (
21).
Our results also provide further insight into possible mechanisms that underlie psychotic symptoms. Many of the striatal functional connections we observed to be significant predictors of treatment response are located in regions that make up the salience network. It has been theorized that the pathophysiology of psychosis is associated with abnormal assignment of salience to external stimuli (
34). Overall, we observed lower striatal connectivity indices in responders and higher indices in nonresponders. Our results indicate that deficits in connectivity between the striatum and regions implicated in various functional networks, including the salience network, may be a target of antipsychotic treatment, consistent with our previous study of treatment mechanisms (
21). By contrast, nonresponders tended to have greater striatal connectivity, suggesting an alternative mechanism for their psychosis that is impervious to the primary functional effects of standard antipsychotic medications. Further studies are required to understand the neurophysiological implications of our results.
While it was not the objective of the present study, our work here opens the door to research that would further characterize our connectivity index. It remains unknown how different stages of psychotic illnesses—from onset to a chronic, relapsing phase—will affect functional connections of the striatum. Similarly, future work may clarify the impact of psychotropic medications other than antipsychotics on our connectivity index. While we find that our striatal connectivity index correlates with length of stay in the hospital, only 11% of the total variance is accounted for by this finding. Further work with additional cohorts will be required to understand this finding.
Limitations of our analysis include access to a relatively limited number of patients in the two cohorts. While we view the heterogeneity of our generalizability data set as a strength of our approach, indicative of the potential for broad applicability, further characterization of the striatal connectivity index in larger, well-characterized cohorts of varying illness subtypes will be necessary before this putative biomarker can be used for clinical decision making. Combinations of this index and other biologically based markers, such as pharmacogenomic measures and genetic loading for illness, may enhance our results. Finally, it will be important to determine how this index works in the context of treatment with clozapine, which has markedly different clinical properties from all other antipsychotics.
To summarize, we describe a potential prognostic biomarker of response to antipsychotic medication in patients entering treatment for psychosis. With further development, the striatal connectivity index may have clinical utility, and with subsequent integration with clinical algorithms available to prescribers, it has the potential to decrease the overall suffering of patients and their families, and to decrease the strain on our health care systems.