A number of studies have reported a significant association between obesity and ADHD (e.g., see references
7–
10), but others failed to confirm this finding (e.g., see references
11–
13). The putative association between ADHD and obesity might seem paradoxical because, rather than being hyperactive, individuals with obesity are often described as “lazy” (
14). However, the impulsivity and inattention that characterize ADHD might lead to dysregulated eating patterns with consequent weight gain (
15). The role of possible confounders, including low socioeconomic status and comorbid mental health conditions, in explaining the association between obesity and ADHD is still unclear (
7,
9,
12,
16). In addition, the role of age, gender, study setting, or study country is also uncertain (
9,
10,
12,
13,
17–
19).
Results
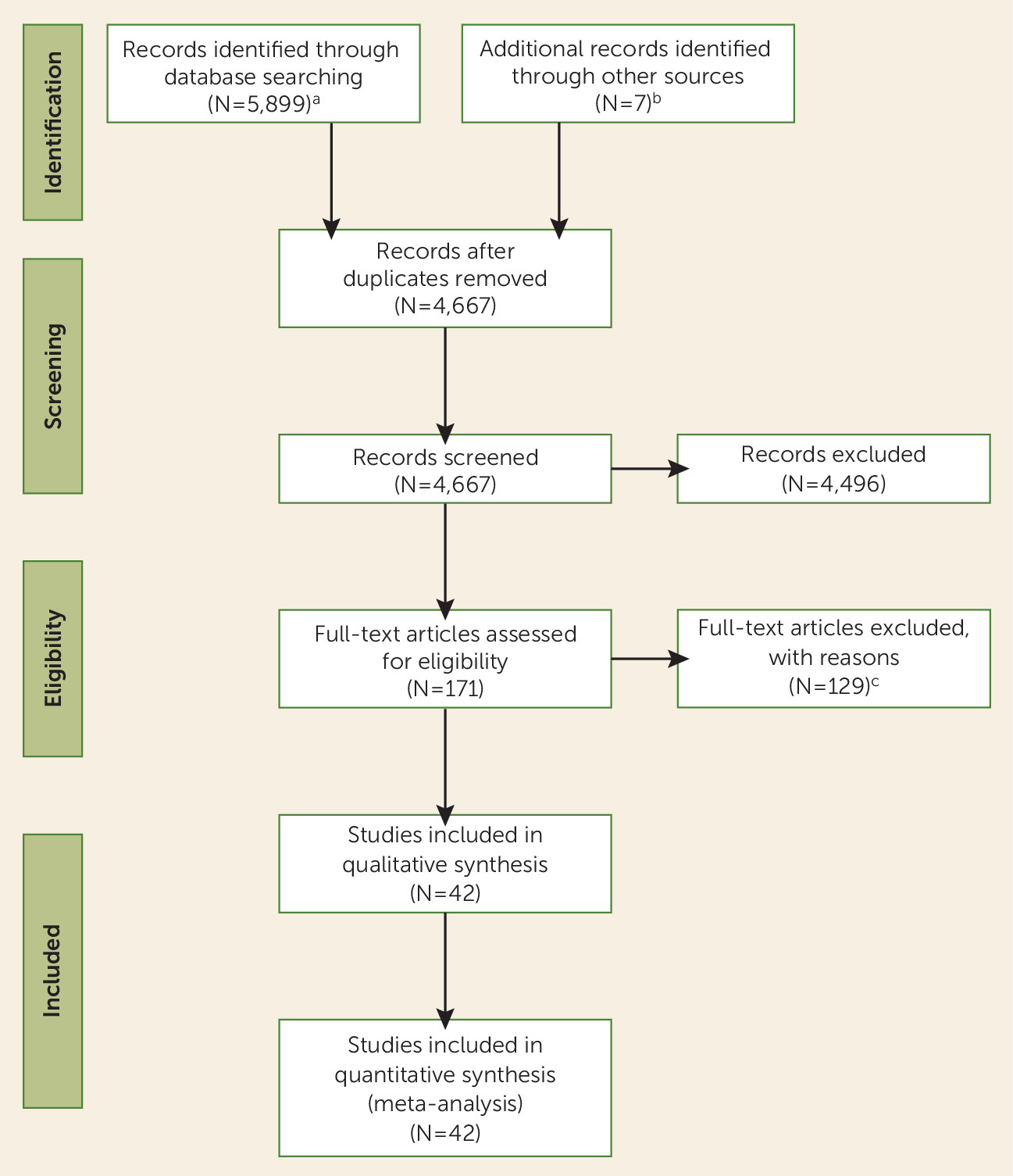
Figure 1 shows the study selection process. We retained 42 studies (
7–
13,
16–
18,
20–
22,
36–
64) for the meta-analysis, comprising 728,136 participants: 48,161 with ADHD (46,115 children; 2,046 adults) and 679,975 comparison subjects (616,228 children; 63,747 adults) (see Table S2 in the online
data supplement). One study (
51) presented data only for overweight rather than for obesity. Studies not included in the meta-analysis after assessment of the full text are listed, with reasons for exclusion, in Table S3 in the
data supplement.
The results of all analyses are summarized in
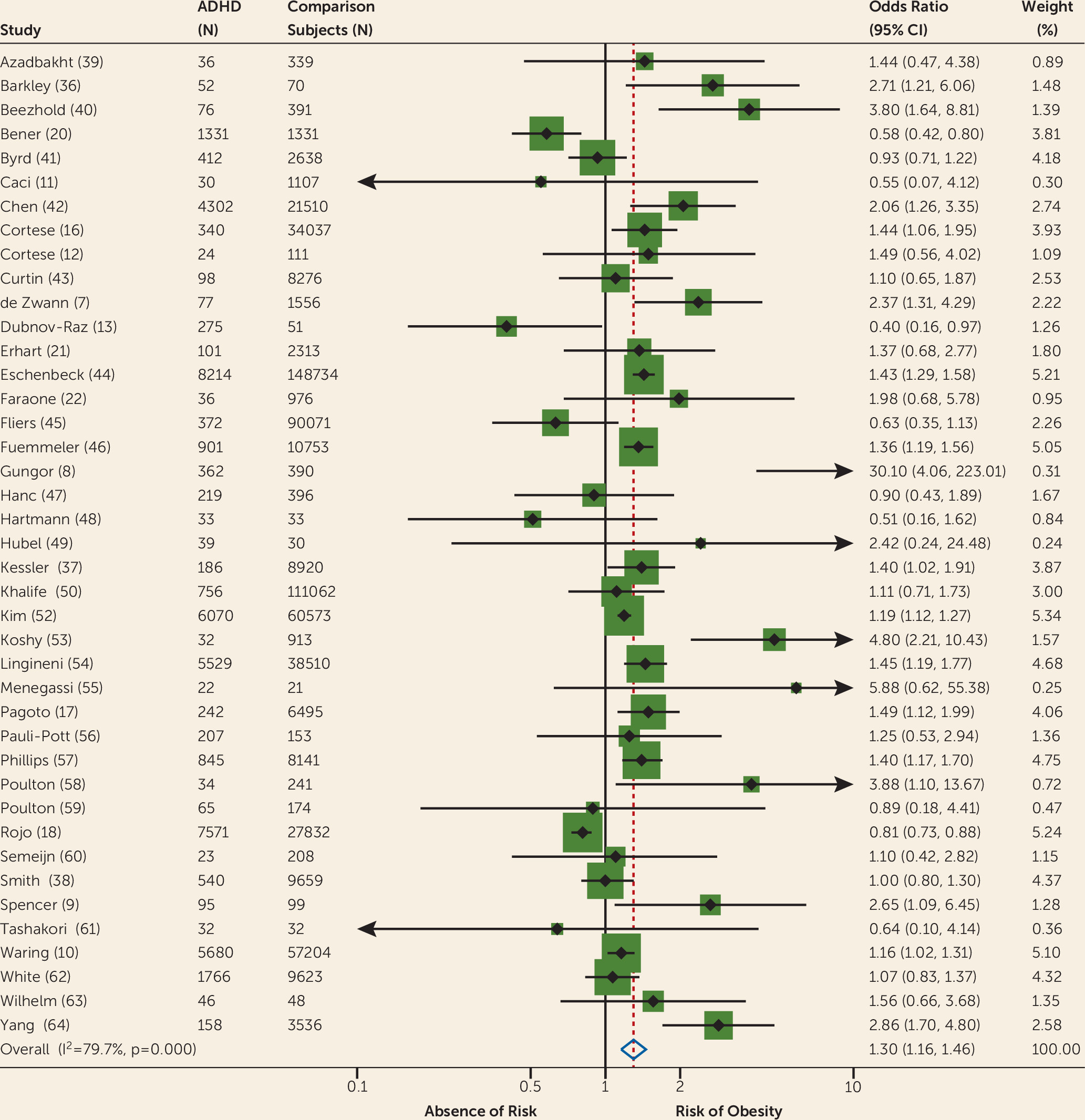
Table 1, which reports tests of heterogeneity and publication bias (Egger’s test). Our first analysis of all available studies (N=41) reporting unadjusted odds ratios for obesity found a significant pooled odds ratio for obesity in individuals with ADHD compared with controls (odds ratio=1.30, 95% CI=1.16–1.46) (
Figure 2). Heterogeneity was high and significant, while Egger’s test was not significant. After removing one clear outlier (
8), results remained significant (odds ratio=1.3, 95% CI=1.1–1.4). Because four studies (
43,
45,
59,
64) used unscreened population-based control groups, which may have included individuals with ADHD, we repeated the analysis without these studies. Results were unchanged (odds ratio=1.3, 95% CI=1.1–1.4).
In both analyses limited to children/adolescents and adults, the pooled odds ratios were significant (odds ratio=1.20, 95% CI=1.05–1.37; and odds ratio=1.55, 95% CI=1.32–1.81, respectively; for further details, see Appendix A3 and Figures S1–S2 in the online data supplement).
The pooled prevalence of obesity was increased by about 70% in adults with ADHD (28.2%, 95% CI=22.8–34.4) compared with adults without ADHD (16.4%, 95% CI=13.4–19.9) and by about 40% in children with ADHD (10.3%, 95% CI=7.9–13.3) compared with children without ADHD (7.4%, 95% CI=5.4–10.1).
Subgroup meta-analyses of studies 1) using a formal diagnosis of ADHD (odds ratio=1.36, 95% CI=1.12–1.66, Figure S3 in the data supplement); 2) with measured height and weight (odds ratio=1.32, 95% CI=1.06–1.66, Figure S4 in the data supplement); 3) in population-based samples (odds ratio=1.24, 95% CI=1.10–1.39, Figure S5 in the data supplement); and 4) in clinical samples (odds ratio=1.61, 95% CI=1.10–2.35, Figure S6 in the data supplement) each found a statistically significant pooled odds ratio (see Appendix A3 in the data supplement). The meta-analysis limited to studies with a formal diagnosis of ADHD and direct measures of height and weight confirmed a significant association between ADHD and obesity (odds ratio=1.47, 95% CI=1.12–1.93, see Figure S7 and Appendix A3 in the data supplement).
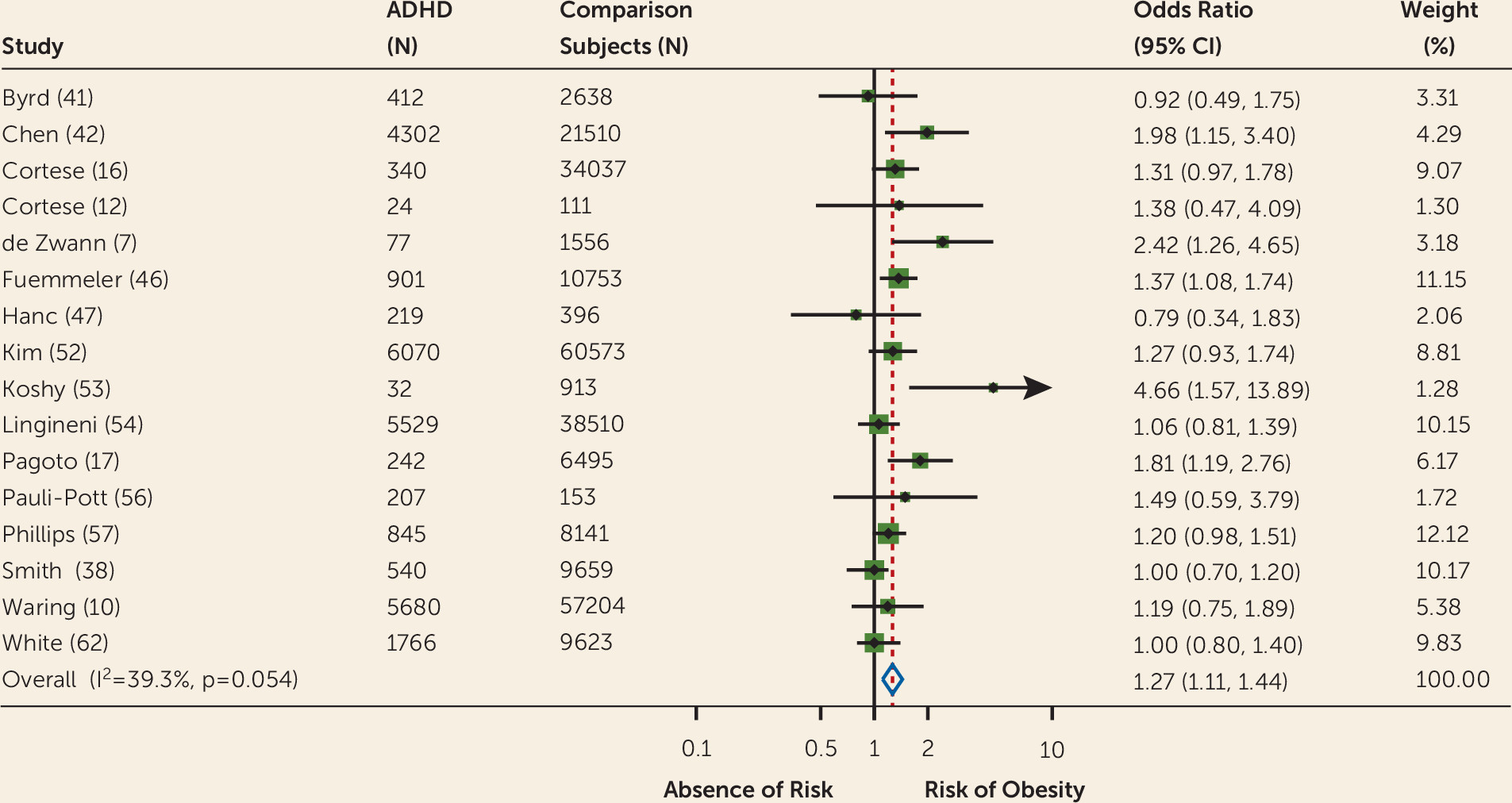
The meta-analysis of adjusted odds ratios found a statistically significant pooled odds ratio (odds ratio=1.27, 95% CI=1.11–1.44,
Figure 3). The heterogeneity and publication bias statistics were not significant.
Meta-regression analysis showed that year of study publication, number of participants with and without ADHD, age group, gender, study setting, and rating on the Newcastle-Ottawa Scale did not significantly influence the pooled odds ratios. The effect of study country, based on a total of 17 countries, was shy of statistical significance (p=0.0506) (see Appendix A3 in the online data supplement).
The meta-analysis of odds ratios for overweight (22 studies) found a statistically significant pooled odds ratio (odds ratio=1.17, 95% CI=1.01–1.36, Figure S8 in the data supplement); heterogeneity was high and significant, while Egger’s test was not significant.
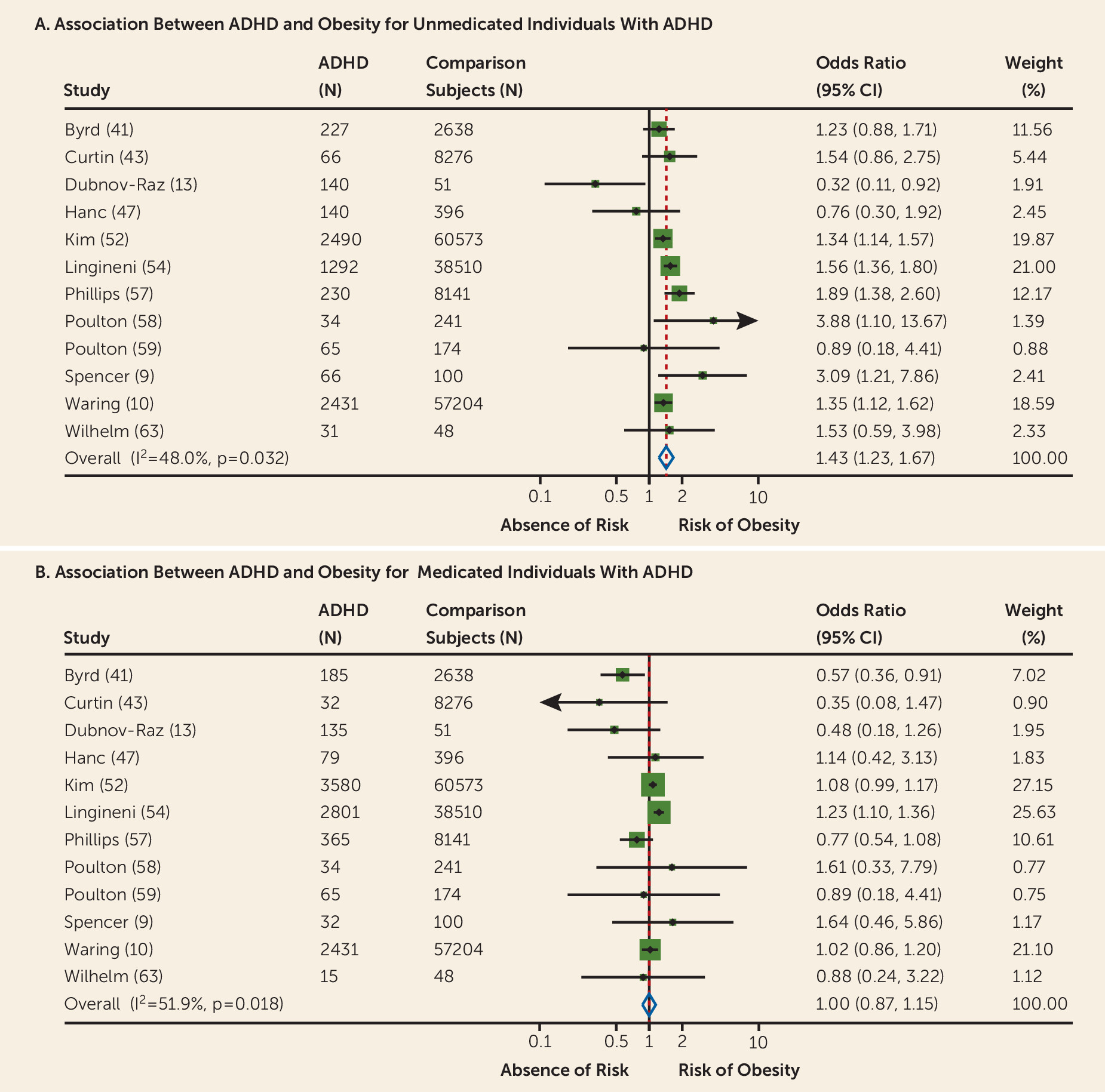
Data on both medicated and nonmedicated participants with ADHD were available from 12 studies. The association between ADHD and obesity was significant for unmedicated (odds ratio=1.43, 95% CI=1.23–1.67,
Figure 4A) but not for medicated (odds ratio=1.00, 95% CI=0.87–1.15,
Figure 4B) individuals with ADHD. Further details are reported in Appendix A3 in the
data supplement.
The pooled prevalence of obesity in participants medicated for ADHD (13.8%, 95% CI=11.4–16.6) was decreased by about 40% compared with those not medicated (19.2%, 95% CI=16.1–22.7).
Discussion
To our knowledge, this is the first meta-analysis assessing the relationship between ADHD and obesity. We found a statistically significant association between these two conditions. The pooled prevalence of obesity was increased by about 70% in adults with ADHD and 40% in children with ADHD compared with subjects without ADHD. These figures, considering the high prevalence of ADHD and the impairment associated with obesity, translate into a conspicuous burden for society.
A significant association emerged also when pooling odds ratios adjusted for possible confounders, including socioeconomic status and comorbid psychiatric conditions (
65). This conclusion should be considered with caution because the type and number of factors adjusted for varied across studies; future research should systematically adjust for a broad set of possible confounders. Special attention should be given to common ADHD comorbid conditions, such as depression, that are also associated with obesity.
Using subgroup meta-analyses, we clarified the role of other factors. We found that the significant association between obesity and ADHD persisted even when focusing only on studies with a formal diagnosis of ADHD, making it unlikely that our results were biased by nonoptimal procedures to detect ADHD, in particular self-reported diagnoses. Another subgroup meta-analysis showed that results did not substantially change when considering only obesity values calculated from directly measured height and weight, suggesting that lower-quality procedures for measuring obesity did not bias our results.
Our meta-regression analysis shed light on the role of additional factors. Prior work has suggested that the association between ADHD and obesity is influenced by gender. One study (
19) of children and adolescents reported a significant association between ADHD symptoms and obesity only in adolescent females; two other studies (
41,
52) found higher odds ratios in unmedicated female adolescents than in male adolescents. According to some authors (
52), higher rates of binge eating in females may mediate this gender effect. But, in contrast to these scattered findings, we showed that gender did not significantly affect the association between ADHD and obesity.
We also found that age group (children/adolescents versus adults) did not influence the association between ADHD and obesity, suggesting that the relationship is already present in childhood. Because only three studies (
43–
45) presented data in children and adolescents separately (for details, see Appendix A4 in the online
data supplement), we could not compare odds ratios in these two developmental periods. It is possible that the significant association between ADHD and obesity in the children/adolescents group was driven either by the primary school group or by the adolescent group. Therefore, further research focusing separately on these two age groups is warranted.
Consistent with our meta-regression analysis, our subgroup analysis showed that the association between obesity and ADHD was similar in clinical and population-based samples; therefore, putative clinical referral biases cannot account for the association.
The effect of study country failed to reach statistical significance. This conclusion should be considered with caution because for the majority of countries only one or two studies were available. Finally, study quality was not a significant predictor in the meta-regression analyses, which is consistent with our subgroup meta-analysis showing the persistence of significant results when including only the most rigorous studies; that is, those with a formal ADHD diagnosis and in which height and weight were measured directly. It is also noteworthy that publication bias was not significant in the majority of the meta-analyses performed.
We also found a significant association between ADHD and overweight, suggesting that future research in the field should examine less severe grades of weight excess in addition to obesity.
In the analyses focused on medication status, we found a nonsignificant association in individuals pharmacologically treated for ADHD, who had rates of obesity that were decreased by about 40% compared with those not treated. These results should be considered with caution because they are based on a subset of studies (N=12), although the total number of participants was still high (9,754 medicated individuals with ADHD; 7,212 nonmedicated individuals with ADHD; 176,352 individuals without ADHD). Importantly, our results based on correlational studies cannot prove that pharmacological treatment for ADHD decreases the risk of obesity associated with ADHD. Overall, there is limited empirical evidence on the short- and long-term effects of psychostimulants on weight specifically focusing on individuals with ADHD and obesity. We are aware of only one naturalistic study (
66) showing that treating comorbid ADHD in adults with a lengthy history of unsuccessful weight loss significantly improved weight loss at 15 months. In that study, appetite suppression vanished within 2 months after the start of the treatment. The study authors argue that the temporary anorexigenic effect of psychostimulants could not account for the observed improvement. Rather, it is possible that the improvements in executive functioning and decreased impulsivity led to more regular eating patterns, with consequent better adherence to diet. Given the scant available research, our results should prompt further investigation on the short- and long-term effects of psychostimulants on weight, aimed at establishing whether weight reduction is causally related to direct metabolic effects of psychostimulants, to long-term normalization of eating patterns, or to other indirect mechanisms. The effects on obesity of nonpharmacological treatments for ADHD should be systematically investigated as well.
The cross-sectional data that we analyzed do not permit firm conclusions about causality in the association between ADHD and obesity. Preliminary longitudinal evidence suggests that ADHD may temporarily precede, and thus contribute to, obesity. A prospective study of 8,106 children (
50) concluded that childhood ADHD symptoms predicted subsequent obesity, rather than the opposite. Another longitudinal study (
12) found that men with a childhood diagnosis of ADHD had a twofold higher rate of obesity compared with those without such a diagnosis. However, because anthropometric data at baseline were not available, the results of that study are equivocal.
Lacking a clear signal from longitudinal studies, several possibilities arise: 1) ADHD could increase the risk of obesity; 2) ADHD and obesity share common biological risk factors, including genetic variants (
67,
68); and 3) obesity or factors associated with it cause or mimic ADHD.
With regard to the first hypothesis, both the impulsive and inattentive components of ADHD could increase the risk of obesity (
15). Deficient inhibitory control, which is an expression of impulsivity and characterizes a large subgroup of individuals with ADHD, could reinforce abnormal eating behaviors that, in turn, would increase the likelihood of obesity (
15). Inattention and poor planning might cause difficulties in adhering to regular eating patterns and dietary regimens (
15); additionally, lack of attention may be associated with lack of awareness of food intake. The hyperactive component of ADHD, as motor overactivity, would intuitively be considered to decrease, rather than increase, the risk of obesity, assuming that it increases energy expenditure and weight loss. However, ADHD motor hyperactivity is not constant but is modulated by the context. For example, it decreases while watching television (
69), and children with ADHD have been shown to watch more television and engage in less physical activity than comparison subjects without ADHD (
52). In addition, hyperactivity linked to restless behaviors may also include abnormal eating patterns. Given the paucity of data, we could not assess the effect of ADHD subtypes, ADHD symptom severity or frequency, and other variables (e.g., watching television, sedentary activity) related to ADHD and obesity. These represent areas of future research for the field.
The hypothesis of common neurobiological dysfunctions in obesity and ADHD reflects the recent notion that many conditions classically thought to be nervous system disorders also include alterations in other physiological systems (
70). ADHD and obesity may share dopaminergic dysfunctions underpinning reward deficiency processing, but this understanding needs to be better evaluated (
71). Interestingly, a “reward deficiency syndrome,” characterized by insufficient dopamine-related natural reinforcement that leads to “unnatural” immediate rewards (such as inappropriate eating), has been reported in both ADHD and obesity (
71). In addition, oxidative stress, which is linked to obesity, has also been associated with ADHD (
72). Moreover, treatment with omega-3 fatty acids, a potent antioxidant, yields significant, albeit modest, reductions in ADHD symptoms (
73).
Finally, it is possible that factors associated with obesity lead to ADHD-like symptoms. Both sleep-disordered breathing (
71) and shorter or later sleep have been reported to manifest with ADHD-like symptoms.
Our results have important clinical and public health implications. The obesity associated with ADHD might explain why patients with ADHD are at increased risk for higher cholesterol levels and higher blood pressure (
9). Assessing the risk for obesity should be part of the assessment and management of ADHD. Clinicians should also screen for ADHD in individuals who are referred for obesity, especially those with a previous history of unsuccessful weight-loss attempts. Although obesity has been found to be associated with other mental health conditions, such as depression (
74) and anxiety (
75), its association with ADHD might be particularly significant for its potential treatment implications.
Our results should be considered in the context of some limitations. First, we endeavored to include unpublished studies, asking experts in the field to provide relevant data, but we could not contact all experts. The inclusion of data from unpublished studies can itself introduce bias due to the willingness of investigators of located unpublished studies to provide data (
18). Second, the definition of obesity in studies in children was based on different BMI thresholds, reflecting a lack of consensus in the field. Third, the exclusion of studies in bariatric samples, although sound, may have led to an underestimation of the strength of the association between ADHD and obesity. Fourth, we focused on obesity/overweight, although a bimodal distribution of weight status in ADHD is possible, whereby ADHD is associated with both obesity/overweight and underweight. This should be assessed in future systematic reviews and meta-analyses. Finally, although we aimed to reduce study heterogeneity by means of subgroup meta-analyses, heterogeneity was still significant in these subgroup analyses. However, in the analysis including studies with adjusted odds ratios, the degree of heterogeneity was not significant.
Acknowledgments
The authors thank the following colleagues for providing additional information on included studies: Ahmad Esmaillzadeh, Ph.D., Sarah Anderson, Ph.D., Bonnie Beezhold, Ph.D., Russel Barkley, Ph.D., Hervé Caci, M.D., Ph.D., Alex Chen, M.D., Martina de Zwann, M.D., Gal Dubnov-Raz, M.D., M.Sc., Eike Eschenbeck, Ph.D., Bernard Fuemmeler, Ph.D., M.P.H., Serdal Gungor, M.D., Ellen Fliers, M.D., Ph.D., Nanda Rommelse, Ph.D., Tomasz Hanc, Ph.D., Andrea Sabrina Hartmann, Ph.D., Alison Poulton, M.D., Alina Rodriguez, Ph.D., Sherry Pagoto, Ph.D., Laura Schieve, Ph.D., Keydra Oladapo, Ph.D., Luis Rojo, Ph.D., Evert Semeijn, Ph.D., Rosemary Tannock, Ph.D., Megan Smith, Ph.D., Russel Viner, M.D., Ph.D., Molly E. Waring, Ph.D., Christine Wilhelm, Dr. Dipl.-Psych., and Beate Herpertz-Dahlmann, M.D., Ph.D. The authors are also grateful to Corrado Barbui, M.D., Ph.D., for advice on methodological issues.