A Novel Strategy for Continuation ECT in Geriatric Depression: Phase 2 of the PRIDE Study
Abstract
Objective:
Method:
Results:
Conclusions:
Method
Design Overview
Participating Centers
Patient Sample
Treatments
ECT plus medication arm.
| Weeks 1–4: Fixed ECT schedule |
|---|
| One treatment 2–5 days after randomization, one treatment 7–12 days after randomization, one treatment 14–19 days after randomization, and one treatment 23–28 days after randomization (a total of four ECT treatments in 1 month) |
| Weeks 5–24: Symptom-Titrated ECT Schedule | |||
|---|---|---|---|
| Treatments per Week | Description | Corresponding HAM-D Condition | Relapse Potential |
| 0 | Current symptom level very low | Current HAM-D score ≤6 | Low |
| or | |||
| Current symptom level low to moderate, with only small drift from baseline level | Current HAM-D score 7–12 and is ≤2 points higher than baseline score | Low | |
| or | |||
| Last two HAM-D scores in remitted range with a flat trajectory (remission stable with less than 2-point change from previous) | Current HAM-D score 7–10 and previous score was 5–10 and current score is ≤2 points higher than previous score | Low | |
| 2 | Current symptom level very high | Current HAM-D score ≥16 | High |
| or | |||
| Current symptom level moderate to high, with trajectory increasing rapidly and large drift from baseline | Current HAM-D score 11–15, and current score is ≥3 points higher than previous score, and current score is ≥8 points higher than baseline score | High | |
| 1 | Patients not requiring 0 or 2 treatments received 1 treatment | Current HAM-D score intermediate between criteria for low or high relapse potential | Moderate |
| Discontinue study | Current and previous HAM-D score ≥21, or the patient is suicidal, or the patient requires psychiatric hospitalization | ||
Medication only arm.
Assessments
Raters
Randomization and Masking of Treatment Assignment
Data Management and Quality Control
Statistical Analysis
Intent-to-treat sample and dropouts.
Missing data.
Efficacy analyses.
Global cognitive functioning and safety analyses.
Results
Sample
| Characteristic | Total Sample (N=120) | ECT Plus Medication (N=61) | Medication Only (N=59) | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 70.5 | 7.2 | 70.8 | 7.2 | 70.3 | 7.3 |
| Education (years) | 14.5 | 3.3 | 14.4 | 3.3 | 14.5 | 3.4 |
| Hamilton Depression Rating Scale (24 item) | ||||||
| Baseline, phase 1 | 30.3 | 7.4 | 29.6 | 6.8 | 31.1 | 7.9 |
| Baseline, phase 2 | 6.1 | 2.5 | 6.0 | 2.5 | 6.1 | 2.5 |
| Mini-Mental State Examination | ||||||
| Baseline, phase 1 | 27.5 | 2.2 | 27.6 | 2.2 | 27.4 | 2.3 |
| Baseline, phase 2 | 27.9 | 2.4 | 27.9 | 2.5 | 27.8 | 2.4 |
| Clinical Global Impressions severity scale | ||||||
| Baseline, phase 1 | 5.2b | 0.9 | 5.1b | 0.8 | 5.3 | 0.9 |
| Baseline, phase 2 | 1.9 | 0.9 | 1.9 | 0.9 | 1.8 | 0.8 |
| Seizure threshold (mC) (baseline, phase 1) | 29.8b | 12.8 | 29.4b | 12.7 | 30.1 | 13.0 |
| Prior antidepressants (baseline, phase 1) | 2.3c | 1.5 | 2.3d | 1.6 | 2.4e | 1.5 |
| N | % | N | % | N | % | |
| Female | 74 | 61.7 | 37 | 60.7 | 37 | 62.7 |
| White | 114 | 95 | 58 | 95.1 | 56 | 94.9 |
| Psychosis (at baseline) | 17 | 14.2 | 5 | 8.2 | 12 | 20.3 |
| Age group (years) | ||||||
| 60–69 | 57 | 47.5 | 29 | 47.5 | 28 | 47.5 |
| 70–79 | 49 | 40.8 | 24 | 39.3 | 25 | 42.4 |
| 80–89 | 14 | 11.7 | 8 | 13.1 | 6 | 10.2 |
| Characteristic | ECT Plus Medication (N=22) | Medication Only (N=26) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 73.3 | 8.1 | 70.4 | 7.3 |
| Education (years) | 13.9 | 3.6 | 13.9 | 3.0 |
| Hamilton Depression Rating Scale (24 item) | ||||
| Baseline, phase 2 | 6.1 | 2.7 | 6.0 | 2.4 |
| Last assessment, phase 2 | 15.9b | 8.5 | 15.7 | 8.2 |
| Mini-Mental State Examination | ||||
| Baseline, phase 2 | 27.3 | 2.7 | 28.3 | 2.0 |
| Last assessment, phase 2 | 27.9c | 2.3 | 27.7 | 3.0 |
| Clinical Global Impressions severity scale | ||||
| Baseline, phase 2 | 1.9 | 0.8 | 1.8 | 0.8 |
| Last assessment, phase 2 | 3.3b | 1.3 | 3.4c | 1.2 |
| ECT treatments in phase 1 | 6.9 | 2.6 | 7.9 | 3.2 |
| N | % | N | % | |
| Female | 13 | 59.1 | 18 | 69.2 |
| White | 21 | 95.5 | 26 | 100.0 |
| Psychosis (at baseline) | 1 | 4.6 | 4 | 15.4 |
Efficacy Analyses
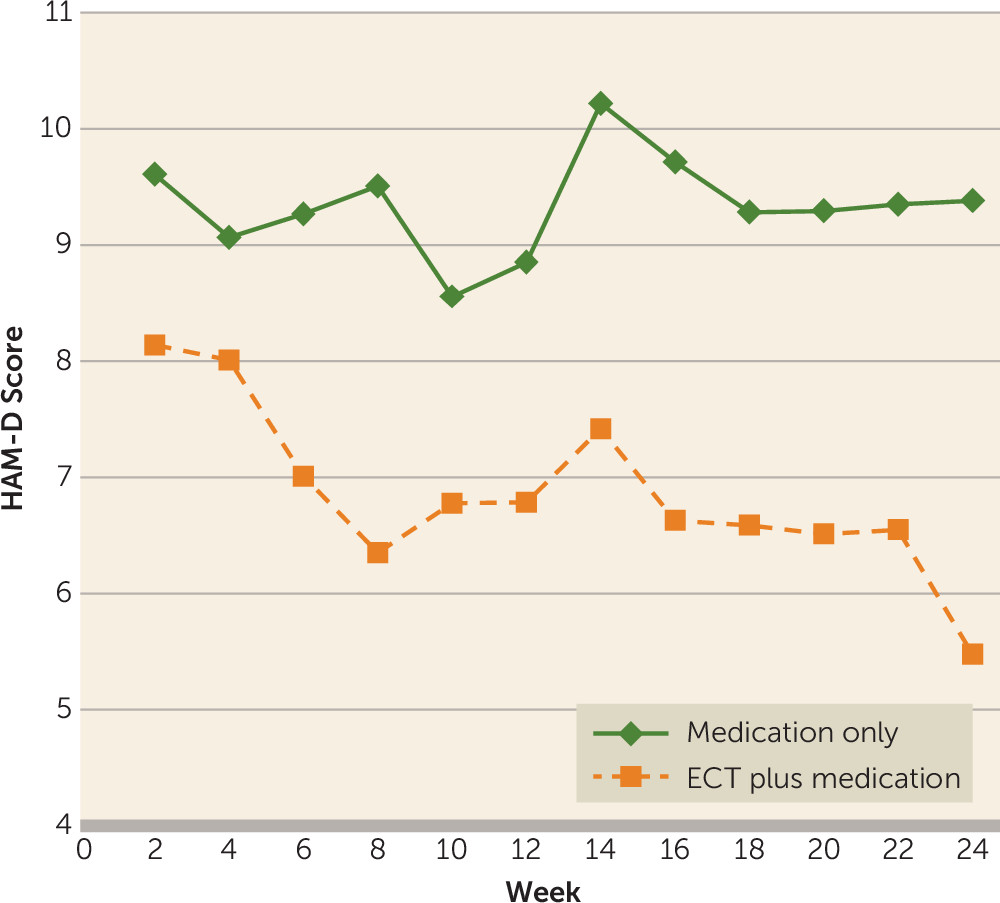
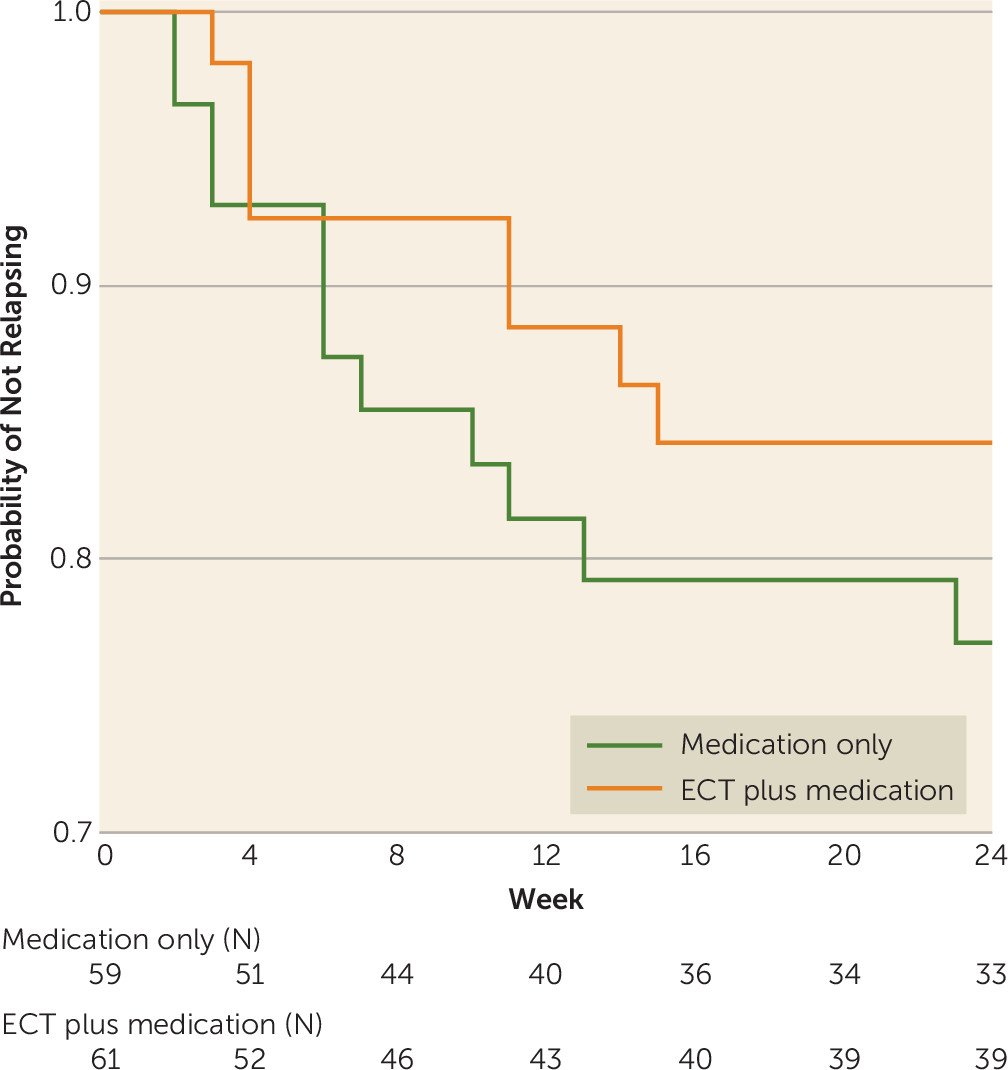
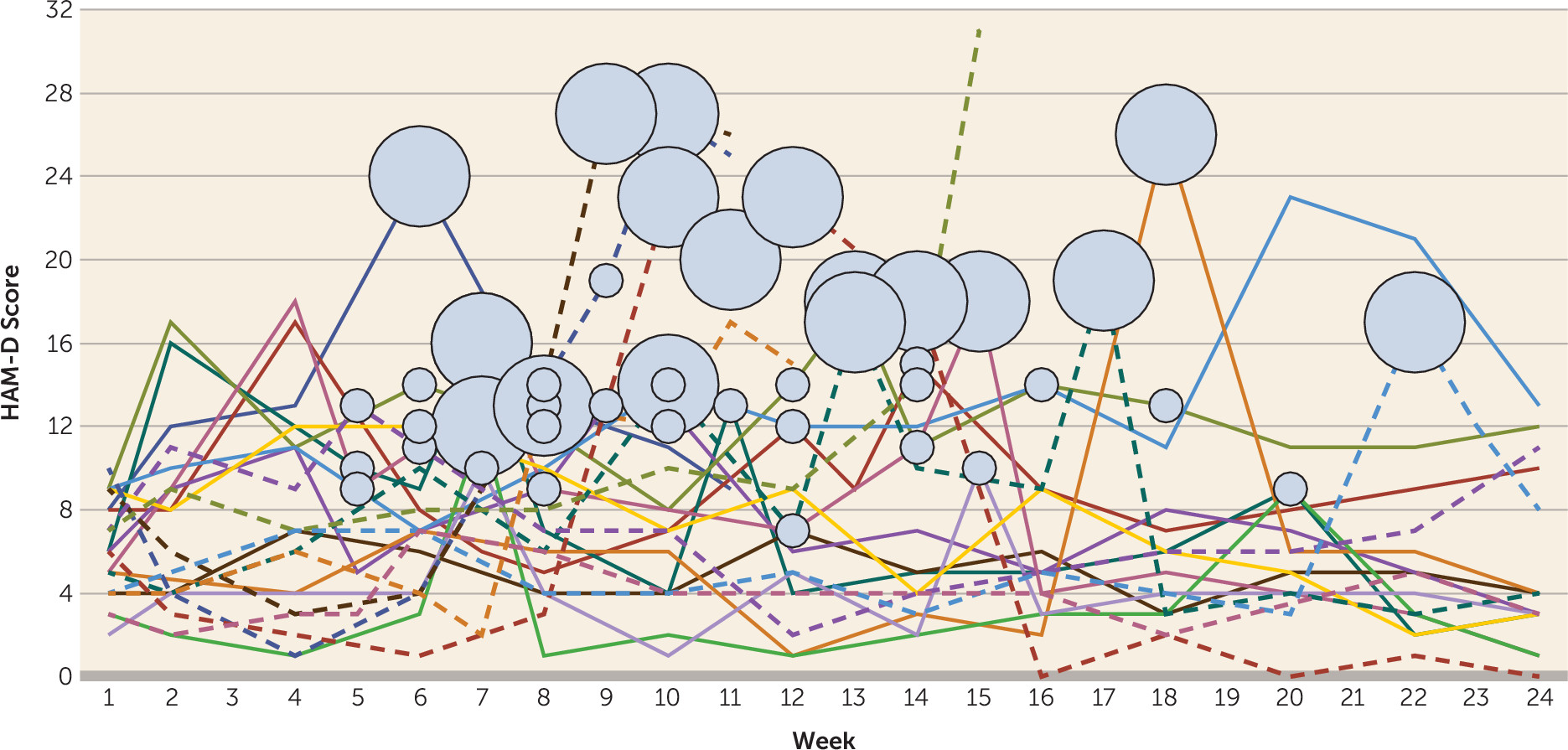
Global Cognitive Functioning
Safety Analyses
Discussion
Footnotes
Supplementary Material
- View/Download
- 410.90 KB
References
Information & Authors
Information
Published In
History
Authors
Author Contributions
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

