Alcohol misuse is the third leading preventable cause of death in the United States and accounts for an estimated $223.5 billion annually (
1,
2), yet 85% of those with an alcohol use disorder never receive treatment (
3). Some populations, such as those with serious mental illnesses, including schizophrenia spectrum, bipolar, and major depressive disorders, are at high risk of suffering from alcohol use disorders, with lifetime prevalence as high as 44% (
4). Among those with serious mental illness, alcohol use negatively affects psychiatric symptoms and contributes to high rates of homelessness, psychiatric hospitalization, HIV infection, cigarette smoking, and drug abuse (
5,
6). Psychosocial treatments demonstrate reductions in alcohol and drug use in adults with serious mental illness (
7,
8); however, few are widely available due to their relatively high costs, need for extensive training and fidelity monitoring, and organizational barriers (
9,
10).
Contingency management is an intervention in which reinforcers, such as vouchers or prizes, are provided, typically multiple times per week, when individuals demonstrate drug abstinence (
11). Point-of-care urine drug tests allow for implementation of contingency management because they are inexpensive, provide immediate results, and verify abstinence for 2 days or more. While contingency management is well established as an intervention for reducing drug use (
11,
12), few studies have investigated contingency management as a treatment for alcohol use disorders (
13–
15). This is primarily due to the lack of an alcohol biomarker that can verify abstinence for 2 days or more. Previous studies have used alcohol breath tests, including mobile video recorded breathalyzers, which can only detect use for approximately 12 hours after ingestion (
13,
15), or transdermal alcohol sensors, which provide continuous monitoring of alcohol use (
14). While these studies have observed reductions in drinking, to date, no randomized trial has investigated the efficacy of contingency management for alcohol use disorders using a biomarker that can verify abstinence for 2 days or more and can be feasibly conducted in a treatment setting.
Ethyl glucuronide (EtG) is an alcohol metabolite produced by the liver that at low cutoffs (100 ng/mL–200 ng/mL) can be detected in urine for up to 5 days after drinking (
16,
17). EtG tests can be conducted in reference laboratories, in clinical settings using a desktop analyzer, or, more recently, using a point-of-care dipcard. We have demonstrated that an EtG immunoassay can be conducted accurately at low cutoffs (100 ng/mL–200 ng/mL) using a benchtop analyzer in an outpatient clinic (
18) and that EtG can be used to implement a contingency management intervention targeting alcohol (
19).
Previous research indicates that contingency management is a feasible and effective intervention for adults with substance use disorders and serious mental illness (
20–
22). In the only large trial (N=176) of contingency management for outpatients with drug dependence and serious mental illness, those who received contingency management for stimulant abstinence were 2.4 times more likely to submit a stimulant negative urine sample during the intervention, compared with those in the control condition (
22). Contingency management participants also reported higher levels of stimulant abstinence during a 3-month follow-up and had lower levels of alcohol use, cigarette smoking, psychiatric symptoms, and inpatient psychiatric hospitalizations, compared with controls (
22,
23). A cost-benefit analysis indicated that these clinical benefits offset the costs of the intervention (
24). Despite these positive findings, a weakness of this study was a contingency management attrition rate of 41%.
The objective of the present study was to determine whether a contingency management intervention that provided reinforcers for alcohol abstinence assessed by EtG and reinforcers for addiction treatment attendance resulted in increased alcohol abstinence in adults with alcohol use disorders and serious mental illness receiving treatment as usual in a community mental health center. Secondary outcomes were other measures of alcohol use, addiction treatment attendance, drug use, psychiatric symptoms, cigarette smoking, and HIV-risk behavior. A prerandomization induction period was used, along with other strategies, to reduce attrition in the randomized sample. To our knowledge, this is the first adequately powered randomized trial of contingency management for alcohol use disorders using an appropriate alcohol biomarker and the second of contingency management in serious mental illness among adults.
Method
Participants
Participants were recruited from a multisite community mental health and addiction treatment agency in Seattle, Wash., and met DSM-IV-TR criteria for alcohol dependence and schizophrenia, schizoaffective disorder, bipolar I or II disorder, or recurrent major depressive disorder assessed by the Mini-International Neuropsychiatric Interview. Other eligibility criteria were alcohol use on >4 days of the last 30 days and enrollment in outpatient addiction group treatment at the agency. Exclusions were comorbid drug dependence, except nicotine, or medical or psychiatric severity that would compromise safe participation.
As described in the CONSORT diagram (see Figure S1 in the data supplement accompanying the online version of this article), 237 individuals were screened for eligibility. Of the 121 eligible participants, 84 successfully completed the 4-week induction period (described below). Five of these individuals were allocated to the contingency management intervention to set the initial magnitude of reinforcement for the noncontingent condition and were not included in the analyses. Seventy-nine participants were randomly assigned to intervention groups and comprised the intent-to-treat sample. Participants provided written informed consent, and procedures were approved by the University of Washington’s Human Subjects Division.
Design and Procedure
This study employed a 4-week induction observation period (weeks 1–4), designed to increase retention after randomization, followed by a 12-week randomized controlled trial of contingency management (weeks 5–16), including a qualitative interview at week 16, with a 3-month follow-up (weeks 17–28). Participants received treatment as usual throughout the study and were randomly assigned to contingency management, where they received reinforcers for EtG-negative urine samples and addiction treatment attendance (N=40), or the noncontingent control group (non-contingent; N=39), in which they received reinforcers regardless of EtG results and treatment attendance. The urn-randomization procedure was used to allocate participants to intervention groups balanced on mood disorder compared with psychotic disorder, level of alcohol use 30 days before baseline, and drug use 30 days before baseline. Randomly assigned participants completed monthly follow-up interviews for 3 months.
Measures
At the baseline interview, participants completed the Mini-International Neuropsychiatric Interview to assess for alcohol use, drug use, and mood and psychotic disorders, and they completed a measure of demographic characteristics. At baseline and weeks 4, 8, 12, 16, 20, 24, and 28, participants completed the Addiction Severity Index-Lite to assess days of alcohol use, drinking to intoxication, and drug use; the Clinical Monitoring Form, Brief Symptom Inventory, and Positive and Negative Symptom Scale for Schizophrenia to assess psychiatric symptom severity; the HIV Risk-Taking Behavior Scale; and the Fagerström Test for Nicotine Dependence. Participants received $20–$30 for these interviews.
Participants provided urine and breath samples three times per week during the induction and intervention and once every 4 weeks during follow-up. The primary alcohol use outcome was urine EtG level as measured by the Diagnostic Reagents Incorporated EtG enzyme immunoassay conducted onsite using a Thermo Fisher Indiko analyzer (Fremont, Calif.). Samples were considered alcohol negative if EtG was <150 ng/mL (
16,
17). Tests were conducted using EtG 100 ng/mL, 500 ng/mL, 1,000 ng/mL, 2,000 ng/mL, and negative calibrators and EtG 100 ng/mL and 375 ng/mL controls. Calibrations occurred weekly. Samples were analyzed on the day of collection. Participants were instructed to avoid nonbeverage sources of ethanol, such as mouthwash and cough syrup. Participants were escorted to the bathroom, and samples were checked for appropriate temperature after collection.
Urine samples were tested for amphetamine, methamphetamine, cocaine, marijuana, and opiate use (Integrated E-Z Split Key Cup II, Innovacon, Inc., San Diego). Breath samples were also tested for carbon monoxide (CO) (Bedfont-Smokerlyzer Micro-IV, Kent, England) (smoking positive CO ≥3 ppm) (
25).
At every study visit, the Alcohol Timeline Followback method (
26) was used to assess hours since the last drinking episode and the number of standard drinks consumed at the last drinking episode in the prior 5 days. These data were used to assess the presence of heavy drinking (women, >3 standard drinks; men, >4 standard drinks) during the prior 5 days. Participants completed a brief qualitative interview assessing the feasibility and effectiveness of the study at week 16.
Study Interventions
Treatment as usual.
Participants were required to be enrolled in state-certified outpatient addiction treatment where they received the same curriculum during group counseling sessions two to four times weekly for 12 weeks or more. This included psychoeducation about the causes of addiction, relapse prevention, and other relevant topics, such as wellness. Participants could receive mental health care, which involved meeting with a case manager once per week and psychiatric medication management, group treatment, and housing and vocational services as appropriate. Self-reported prerandomization utilization of addiction services did not vary by group.
Induction period.
After the baseline interview, participants entered a 4-week induction where they received reinforcers contingent on providing urine samples three times per week regardless of EtG results. Participants received three “prize draws” (described below) for each urine sample provided. The induction period has been used in previous studies to decrease attrition in the randomized sample by selecting for randomization only those who can attend brief study appointments and are currently using alcohol (
27,
28). Participants who attended at least one visit during the fourth week and provided at least one EtG-positive urine sample at any time during induction were randomly assigned. Individuals who did not initially meet criteria for randomization were allowed to re-enroll in the study. Re-enrolled individuals completed a second baseline and induction period. Ten participants were re-enrolled, and six were randomly assigned.
Contingency management.
Participants in contingency management received the variable magnitude of reinforcement procedure each time they tested negative for EtG. This well-researched procedure involves making “prize draws” from a container of tokens representing different magnitudes of reinforcement (
11). Fifty-percent read “good job” (no prize). The other 50% were associated with a tangible prize (41.8% read “small” with a $1.00 value; 8.0% read “large” with a $20.00 value; and 0.2% read “jumbo” with an $80.00 value).
Participants provided urine samples three times per week for 12 weeks. They earned at least three prize draws for each EtG-negative urine sample submitted (EtG <150 ng/mL). One additional prize draw was earned for each week of continuous alcohol abstinence (three consecutive EtG-negative samples). Participants who missed an appointment or had an EtG-positive sample did not earn prize draws and had a “reset” to three prize draws when their next negative sample was submitted. Following a reset, participants could return to the number of prize draws at which the reset occurred by providing three consecutive EtG-negative samples. Participants received gift cards for attending all ($10.00) or at least one ($5.00) of their addiction groups each week.
Noncontingent reinforcement.
Noncontingent participants submitted urine samples three times a week for 12 weeks but received prize draws for each urine sample submitted, regardless of EtG results. Consistent with previous studies (
22,
29), the number of prize draws they received was equal to the average number of prize draws earned by the contingency management group in the previous week. This procedure assured that groups received an equal number of prize draws. Noncontingent participants did not need to attend addiction treatment to earn gift cards; instead they received gift cards equal to those earned by the contingency management group during the previous week.
Reinforcers and earnings.
The total average values of prizes earned by the noncontingent (mean=$147.54 [SD=$143.19]) and contingency management (mean=$175.03 [SD=$183.17]) groups were not significantly different.
Attrition.
Individuals who missed nine consecutive study visits (3 weeks) during the intervention phase were considered dropouts. These individuals could complete follow-up interviews and were included in the intent-to-treat analyses.
Data Analysis
We used chi-square tests for categorical variables and t tests for continuous variables to examine baseline differences across groups. Analyses were conducted on the intent-to-treat sample. Generalized estimation equations were used to analyze outcomes that were collected over time. The maximum likelihood estimation procedure was used (
30). To protect against type-I error, analyses utilized bidirectional tests despite our unidirectional hypotheses. Odds ratios with 95% confidence intervals are presented for binary outcomes, and unstandardized regression coefficients with 95% confidence intervals are presented for continuous outcomes. These methods of analysis have been used in many contingency management trials and are effective and efficient methods of analyzing outcomes across time nested within participants (
22,
29). Analyses controlled for baseline levels of the outcome being analyzed.
Multiple imputation procedures were used to handle missing data. This approach has advantages over single imputation, listwise deletion, or other techniques (
31,
32) in conjunction with generalized estimation equation analyses. We present listwise deletion analyses first and include the multiple imputation analyses in Table S1 in the
online data supplement to provide confidence bounds for the location of the true treatment effect. Preliminary analyses identified several variables that predicted missingness due to intervention dropout. We used these variables during the imputation phase to ensure our “missing at random” assumption was tenable. While there is no test for whether missing data are truly “missing at random” compared with “missing not at random,” our inclusive strategy for auxiliary variables during imputation made for a tenable assumption that data were “missing at random.” Multiple imputation procedures used a regression-based approach to fill in the missing values to produce multiple datasets. Fifty datasets were analyzed for each analysis to maximize statistical efficiency. Parameters and standard errors were combined using Rubin’s rules (
33). Analyses were performed using Stata 14.1 (StataCorp, College Station, Tex.).
Results
Twenty-six (65%) contingency management and 29 (74%) noncontingent participants completed the intervention phase, a nonsignificant difference. Thirty (75%) of the contingency management participants and 30 (77%) of the noncontingent participants attended at least one of three follow-up assessments, and 42 (53%) participants attended all follow-up assessments. Demographic and clinical characteristics of the sample did not differ by group (see
Table 1).
EtG and Self-Reported Alcohol Use
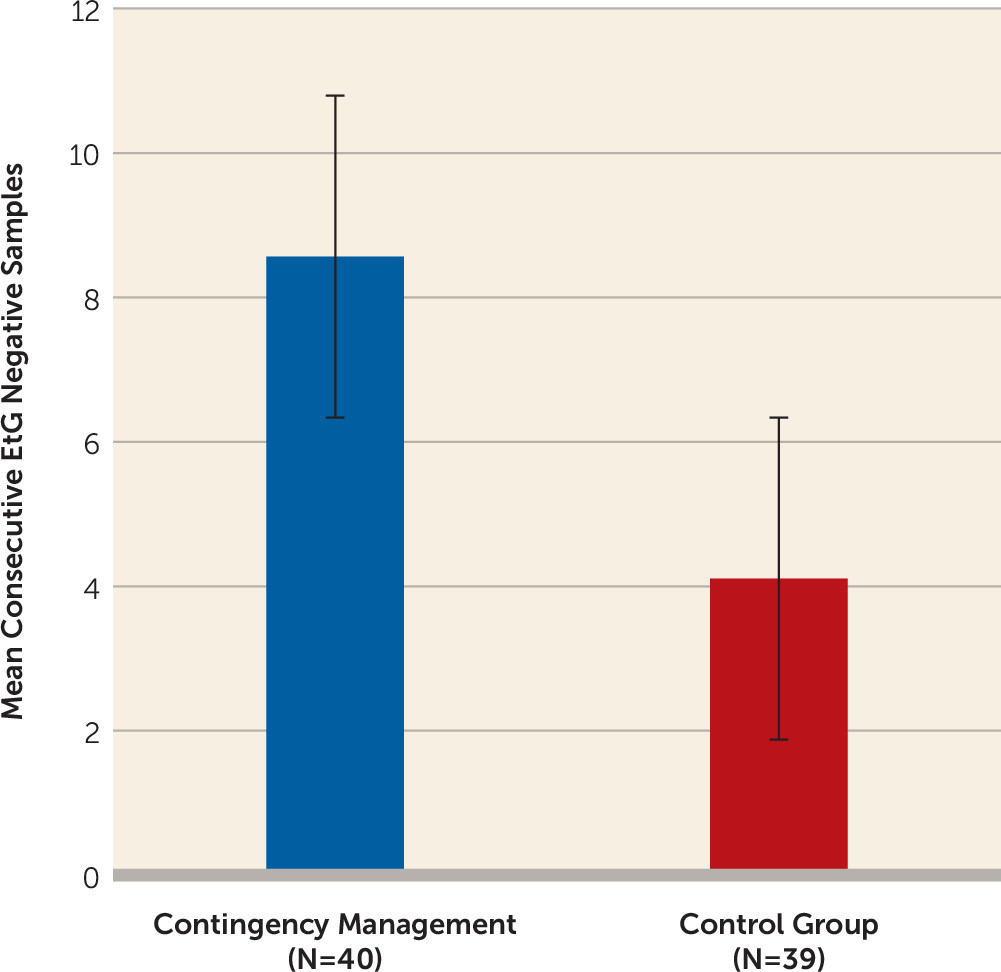
As
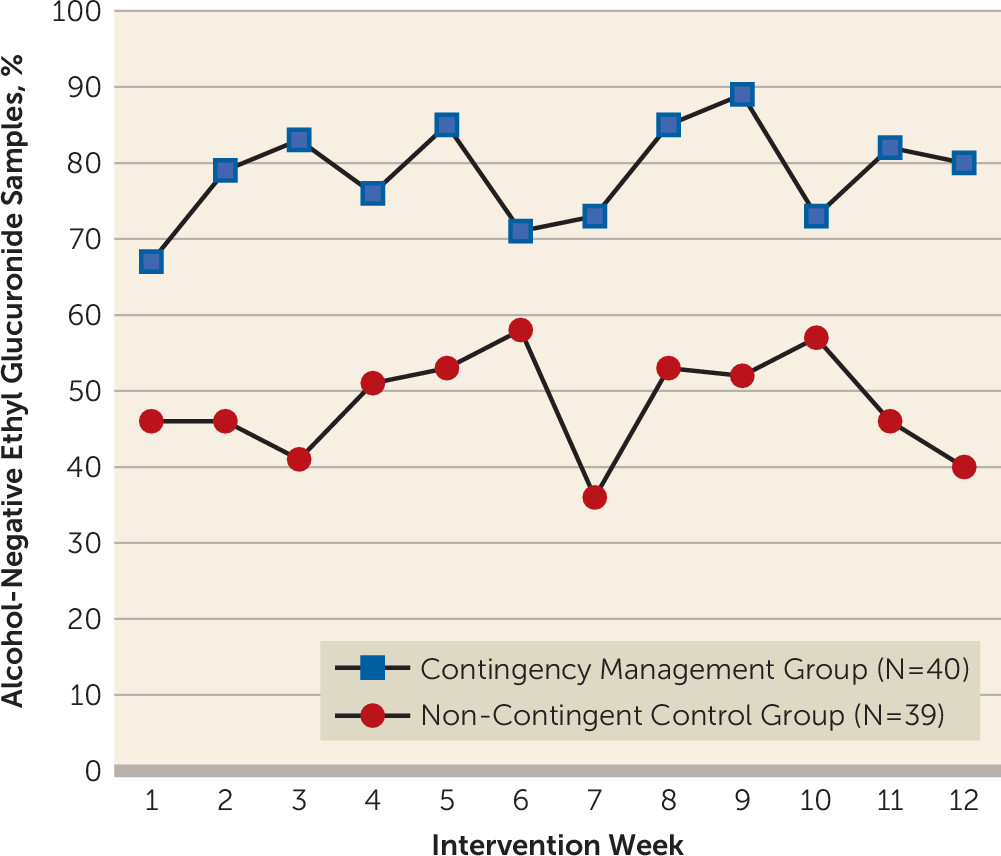
Figure 1 illustrates, individuals randomly assigned to contingency management had a mean longest duration of alcohol abstinence (number of consecutive EtG-negative urine samples) more than twice as long as the mean for controls (contingency management: mean=8.56 [SD=12.59]; noncontingent: mean=4.11 [SD=1.22]; F=5.55, df=1, 76, p<0.05), with approximately 1.5 weeks of additional abstinence. Contingency management participants were 3.13 times (95% confidence interval [CI]=2.18–4.50, p<0.05) more likely to submit an EtG-negative urine sample during the intervention period compared with controls (see
Figure 2). Contingency management participants had significantly lower EtG levels during the intervention period compared with controls (B=325.93; 95% CI=213.35–438.51, p<0.05). While group differences on both the binary and continuous versions of EtG persisted into follow-up (see
Table 2), these differences were nonsignificant. Multiple imputation analyses verified results of EtG analyses during intervention and follow-up (see Table S1 in the
online data supplement).
Contingency management participants had significantly fewer days of any drinking (B=8.29; 95% CI=3.97–12.60, p<0.05) and drinking to intoxication (B=6.43; 95% CI=2.40–10.47, p<0.05) throughout the intervention period, compared with controls (see
Table 2). They were 3.48 times (odds ratio=3.48; 95% CI=2.32–5.23, p<0.05) less likely to report recent heavy drinking during the intervention period compared with controls. These findings persisted into the follow-up period (days of drinking: B=7.40, 95% CI=3.52–11.29, p<0.05; days of intoxication: B=5.69, 95% CI=2.57–8.80, p<0.05; heavy drinking in last 5 days: odds ratio=4.90, 95% CI=1.78–13.47, p<0.05). These differences were significant using the multiple imputations during treatment and follow-up (see Table S1 in the
online data supplement).
Secondary Outcomes
Contingency management participants were more likely to submit stimulant-negative urine tests during the intervention (odds ratio=3.19, 95% CI=1.99–5.10, p<0.05) and follow-up periods (odds ratio=4.59, 95% CI=1.24–17.08, p<0.05) and self-reported fewer days of stimulant drug use during the intervention period (B=2.22, 95% CI=0.34–4.10, p<0.05). Multiple imputation analyses found similar results for stimulant drug tests (see Table S1 in the online data supplement). The multiple imputation analyses replicated the effect size of self-reported days of stimulant drug use, but the treatment effect for the intervention period was not significant (see Table S1 in the online data supplement).
Among the 54 smokers identified by smoking-positive self-report or carbon monoxide samples at baseline, contingency management participants were 5.4 times more likely to submit a smoking-negative breath sample, compared with controls, during the intervention period (odds ratio=5.39, 95% CI=1.93–15.0, p<0.05). The multiple imputation model represented an attenuation of these findings (see Table S1 in the online data supplement). This difference was not significant during the follow-up period. Group differences on other secondary outcomes including addiction treatment attendance, opioid and marijuana use, psychiatric symptoms, HIV-risk behavior, and self-reported cigarette smoking were not statistically significant.
Discussion
To our knowledge, this is the first adequately powered randomized trial of contingency management for alcohol use disorders utilizing an accurate alcohol biomarker. Those who received contingency management were three times more likely to submit an EtG-negative urine sample during the intervention period, and they attained approximately 1.5 additional weeks of alcohol abstinence compared with controls. Those who were assigned to contingency management had an average EtG level that was approximately 300 ng/mL lower than controls during the intervention period. These differences are large and clinically significant. Self-report data corroborated EtG results, with contingency management participants reporting fewer days of alcohol use, drinking to intoxication, and episodes of heavy drinking during the intervention and follow-up periods. Results are consistent with reductions in drinking during contingency management (
13–
15), as well as during follow-up (
14) reported by others. This study provides further evidence of postintervention benefits of contingency management for alcohol use (
14).
This is the second trial to investigate the efficacy of a contingency management intervention focused on promoting abstinence from alcohol or drug use in adults with serious mental illness (
22). Both trials demonstrated that contingency management is a powerful procedure for reducing substance use when added to treatment as usual at a community mental health center. This study also replicates our previous findings of group differences in nontargeted drug use and cigarette smoking (
22,
23). We did not observe group differences in other outcomes, such as psychiatric functioning or HIV-risk behavior. However, this could be due to a lack of statistical power, given the relatively small sample size.
The contingency management completion rate (65%) was superior to our previous study in adults with serious mental illness and stimulant dependence (41%) (
22) and is comparable, if not superior, to contingency management studies in populations without serious mental illness (
29). We implemented a number of strategies to reduce attrition, including requiring participants be in treatment as usual, increasing the minimum number of prize draws for each abstinent urine sample from one to three, and utilizing a four-week induction period prior to randomization. While differences in completion rates could be due to differences in study populations, and the first two strategies may have reduced attrition, we hypothesize that the induction period played an important part in the improved contingency management completion observed in this study. Aside from the methodological advantages, the induction period may have clinical relevance. It allows clinicians to identify individuals who are most likely to benefit from contingency management (i.e., current alcohol users who can attend frequent brief intervention visits) and allows individuals to receive reinforcers before abstinence is required. Those who cannot complete the induction are unlikely to benefit from contingency management but might benefit from other interventions.
Limitations of this study include the relatively small sample size, which may have limited our ability to observe statistically significantly differences during follow-up, as well as differences in psychiatric symptoms. Additionally, while we observed reductions in smoking, we did not observe group differences in smoking quit rates. Recruitment from one agency and the use of the induction period to restrict randomization to individuals best suited to contingency management might limit the generalizability of study results. In terms of age (mean=45.38 [SD=10.20]) and gender (37% female), the sample is consistent with other studies of those with co-occurring disorders (
21). Another limitation was the use of a $30,000 onsite urine analyzer to conduct EtG tests, and an additional approximate cost of $2.50 per test due to chemicals required for testing. The recent development of inexpensive ($4.00) point-of-care EtG immunoassay dipcard tests (Confirm Biosciences, San Diego) further improves the feasibility of an EtG-based contingency management intervention. Funding for reinforcers and urine tests can be a barrier to implementation. However, this barrier is likely surmountable as cost-effectiveness studies of contingency management in those with and without serious mental illness support the intervention’s cost-savings (
24,
34,
35).
Results of this study strongly support the efficacy of an EtG-based contingency management intervention for alcohol use disorders. Group differences in alcohol use observed in this study and positive outcomes of our previous trial of contingency management targeting stimulant use suggest that contingency management may be a particularly effective intervention for those with co-occurring serious mental illness and substance use disorders, a high-cost and difficult-to-engage population.
Acknowledgments
The authors thank the leadership and staff at Community Psychiatric Clinic, including Shirley Havenga, Kelli Nomura, Susan Peacy, Liz Quakenbush, Kurt Davis, and all those who volunteered to participate in this study.