There is evidence of a continuity between clinical and subclinical phenotypes of psychosis that is measurable in the general population (
1) and in individuals with a psychiatric diagnosis (
2). On a clinical level, individual differences in psychosis proneness are expressed across a number of psychiatric conditions besides schizophrenia, namely mood, anxiety, eating, impulse control, and substance use disorders (
2). At a subclinical level this liability is characterized by “attenuated” or “brief” psychotic symptoms that might not coexist with other diagnostic criteria (frequency and intensity) to meet the full diagnosis, yet sufficient impairment is observed to motivate treatment seeking (
3). This clinical high-risk state has been shown to be a robust risk factor for progression to clinically significant psychiatric disorders (
4) but does not necessarily predict one specific disorder and instead is predictive of a number of psychopathologies that include psychotic symptoms (
5).
At the far end of the extended psychosis continuum are children and adolescents from the community who report psychotic-like experiences (i.e., perceptual abnormalities and delusional thoughts) prior to the onset of more-impairing psychotic symptoms. These preclinical experiences, even though they are common in children and young adolescents (7% to 23% [
6]), are associated with increased risk for psychotic or other axis I disorders over the longer term (
6,
7). Studying young adolescents prone to such experiences will help to identify etiologic processes implicated in psychosis proneness, without the confounds of diverse risk factors and iatrogenic effects, such as substance misuse, medication, and social impairment (
8). Investigating the neural correlates of this preclinical psychosis proneness during cognitive functioning can shed light on early altered neural processes prior to significant cognitive impairments.
However, the vast majority of functional magnetic resonance imaging (fMRI) studies have focused on adults with a clinical risk for psychosis, not on young adolescents reporting psychotic-like experiences. These studies have mostly investigated the neural circuits implicated in executive functioning, social cognition, and reinforcement learning. Recent fMRI studies in individuals with psychosis spectrum symptoms have shown significant reduced activation in the dorsolateral prefrontal cortex (DLPFC) during executive functioning (e.g., working memory, inhibitory control) relative to low-risk control subjects (
9,
10). These results are consistent with findings of DLPFC hypoactivation in patients with clinical diagnoses of psychosis or bipolar disorder during tasks involving working memory and response inhibition (
11,
12), suggesting that the brain markers associated with psychosis proneness cross diagnostic boundaries.
Social cognition, which encompasses emotion processing and theory of mind processes, has also been identified as a domain that might differentiate individuals at clinical risk for psychosis from low-risk individuals (
13). Neuroimaging studies show that the experience of high-arousal negative emotions is associated, in individuals at clinical risk relative to healthy control subjects, with both reduced (
14) and increased (
9) activation of frontolimbic areas, depending on the contrast used (
15), while viewing neutral material is more consistently associated with increased activation of this network (
14,
16).
Another core feature of psychosis, dysfunctional reinforcement learning, has been shown to be shared with distinct diagnostic categories, such as major depressive and bipolar disorders (
17). A recent meta-analysis of fMRI studies demonstrated that psychosis spectrum disorders are associated with a blunted response from the ventral striatum during anticipation of reward, which might explain why patients manifest impaired learning of stimulus-reinforcement associations (
18). Functional MRI studies with clinically at-risk individuals have shown modestly reduced activity in frontostriatal regions during reward anticipation, relative to control subjects (
19,
20).
Among the very few neuroimaging studies investigating the early neural correlates of preclinical psychosis proneness prior to the onset of more-impairing psychotic symptoms, those by Modinos et al. (
21,
22) showed that community youths self-reporting psychotic-like experiences had reduced activation of the medial prefrontal cortex, insula, and amygdala during passive viewing and reappraisal of negative pictures relative to low-risk youths. In a quite young sample of 11- to 13-year-olds reporting these experiences, Jacobson et al. (
23) observed reduced activity in prefrontal and temporal regions during a response inhibition task. However, the sample was small (11 in the at-risk group). Consequently, we intended to extend these findings in another community sample of young adolescents with psychotic-like experiences. Of note, considering that psychotic-like experiences are, for most individuals, transient and not persistent (
1), it is crucial to understand to what extent these early neural abnormalities relate to a subsequent psychosis vulnerability in terms of clinically validated symptoms.
The primary aim of the present exploratory study was to identify brain correlates of psychotic-like experiences in youths prior to exposure to regular substance use, by means of fMRI measures of emotion processing, inhibitory control, and reward anticipation. The data are from the IMAGEN study, in which two sites, London and Dublin, assessed these preclinical experiences in participants when they were 14 years old. The secondary aim was to validate whether these brain correlates predicted emergence of psychotic symptoms in the context of mood fluctuation symptoms at age 16 in the full IMAGEN sample. We hypothesized that psychotic-like experiences would be associated with reduced activity in the executive network during response inhibition, altered activity in frontolimbic regions during processing of emotional and nonemotional stimuli, as well as modest reductions in ventral striatum activity during anticipation of reward.
Method
Participants
In the large European multicenter IMAGEN study, 2,257 14-year-old adolescents were recruited through high schools from eight sites across the United Kingdom, Ireland, France, and Germany. Parents and adolescents gave written informed consent to the study procedures. All procedures were approved by each local institutional ethics committee. A detailed description of the study recruitment and assessment procedure, exclusion criteria, data storage and safety, as well as imaging acquisition protocol may be found elsewhere (
24).
Measures
For a more detailed description of the study measures, see the data supplement accompanying the online version of this article.
Psychotic-like experiences.
At baseline, the 14-year-olds from London and Dublin completed the self-report Adolescent Psychotic-Like Symptoms Screener (
25), which contains seven items evaluating perceptual abnormalities and delusional thoughts in the past 6 months. Participants were asked to rate their responses to different statements on a 1-point scale (0=not true, 0.5=somewhat true, 1=certainly true). Based on previous studies by Cannon’s team (
23,
25), to identify youth with significant psychotic-like experiences, we used the following criteria: a total score ≥2 and a score ≥0.5 on the auditory hallucination item (this item revealed 88% probability of predicting which individuals would be classified as “at risk,” as determined by consensus ratings from the Structured Interview for Prodromal Syndromes).
Among 410 adolescents from the London and Dublin sites (mean age=14.3 years, SD=0.4; 51.7% girls), 300 had complete fMRI and behavioral information. Among them, 27 were classified as having significant psychotic-like experiences. None had yet started using cannabis, and they reported minimal alcohol and cigarette use (<3–5 times in the previous year). By means of an in-house groupwise matching script designed by the IMAGEN consortium, the group was matched (on sex, handedness, imaging site, general IQ, and puberty development) to a control group five times as large (135 adolescents), who had a total score ≤1 and a score of 0 on the auditory hallucination question.
Psychotic symptoms at age 16.
For the secondary objective of the study, psychotic symptoms were evaluated with the self-report Development and Well-Being Assessment interview (
www.dawba.com) (
26), a computer-based package of questionnaires designed to generate DSM-IV-TR psychiatric diagnoses for 5- to 16-year-olds. The schizophrenia module was not administered to participants at age 16; the bipolar module was more developmentally appropriate for this age group. Therefore, all participants answered initial screening questions assessing mood dysregulation (“rapid mood changes” and “abnormally high mood”), and if they gave a positive response, they were then asked three specific items assessing the presence of visual and auditory hallucinations and delusional beliefs. Among the 300 individuals from London and Dublin with complete baseline assessments, 246 (82.0%) completed the bipolar module at age 16 and were further divided into three groups: those who endorsed mood dysregulation plus hallucinatory/delusional symptoms (i.e., group with mood and psychotic symptoms, N=12), those reporting mood dysregulation without hallucinatory/delusional symptoms (i.e., group with mood symptoms only, N=80), and those who did not endorse the mood dysregulation criteria (i.e., no mood symptoms group, N=154).
Additionally, we conducted similar analyses on the full IMAGEN sample. Among the 1,602 participants reassessed at 16 years old, 1,196 had complete fMRI and behavioral information and were divided into three groups: those with mood and psychotic symptoms (N=72), those with mood symptoms only (N=451), and those without any mood symptoms (N=673).
Neuroimaging tasks.
We report results from three task-based fMRI paradigms: 1) the faces task, to assess emotional processing, 2) the stop-signal task, to evaluate motor inhibitory control, and 3) a modified version of the monetary incentive delay task, to examine reward anticipation. The block-design faces task, known to elicit prefrontal and amygdala activations (
27), uses video clips displaying a neutral expression progressively turning into an angry or a second neutral expression. A control condition displays expanding/contracting circles. In the event-related adaptation of the stop-signal task used to measure activation of the frontostriatal network (
28), a motor response to high-frequency go signals (83.3% of trials) has to be inhibited when, infrequently and unexpectedly (in randomized 16.7% of trials), a stop signal appears after the go signal. In the modified monetary incentive delay task, participants had to respond to a target in order to win a previously indicated amount of points (three trial types: no win, small win, and large win). In the anticipation phase, which elicits striatal and medial prefrontal activity (
29), participants were presented with cues signaling the amount of reward that could be won in a given trial.
Data Analysis
fMRI.
To test differences in brain activity between the groups reporting and not reporting psychotic-like experiences on each of the contrasts of interest (faces: angry versus neutral and neutral versus control; stop-signal: stop success versus baseline and stop failure versus baseline; monetary incentive delay: anticipation of large reward versu
s no reward), we conducted two-sample t tests, using a whole-brain approach in SPM8 (Wellcome Trust Centre for Neuroimaging,
http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Following the recommendations of Eklund et al. (
30) for controlling type 1 error, we used the new version of AFNI’s 3dClustSim (
https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html) to calculate a voxel-wise threshold correcting for multiple comparisons and controlling for family-wise error rate. Significant voxels were required to be part of a cluster of more than 24 contiguous voxels, giving a 0.05% probability of a cluster surviving due to chance. For our secondary objective, we used a more liberal threshold: significant voxels were required to be part of a cluster of at least 10 contiguous voxels. Then we created regions of interest based on the regions’ coordinates and extracted the mean contrast value (betas) for each region of interest and for each subject.
Machine learning procedure.
For our secondary objective, we aimed to classify the youths according to the 16-year-olds’ psychotic outcomes with fMRI information. We conducted cross-validated logistic regressions with elastic-net regularization to model this relationship. Cross-validation is used to evaluate how well a predictive model generalizes to out-of-sample observations. On one hand, leave-one-out cross-validations were used during classification of the groups within the smaller London-Dublin subsample; on the other hand,
k(10)
-fold cross-validations were used during classification of the groups within the full sample. Cross-validation analysis within the London-Dublin subsample allowed testing of the predictive capacity of the brain markers while controlling for baseline psychotic-like experiences. Considering that the sample size of the groups was much larger in the full sample, we were able to control for more predictors, such as developmental risk factors for psychotic symptoms (i.e., cannabis, alcohol, and cigarette use, as well as internalizing and externalizing behaviors [
31]), assessed at age 14.
Elastic-net regularization is used to achieve better prediction performance by penalizing the regression coefficients in an attempt to minimize overfit. Elastic-net regularization is an example of a sparse regression method, which imposes a hybrid of both L1- and L2-norm penalties (i.e., penalties on the absolute [L1 norm] and squared [L2 norm] values of the regression coefficients). Model performance was evaluated by using the area under the curve (AUC) of the receiver operating characteristic (ROC), which quantifies the predicted sensitivity (true positive rate) as a function of the false positive rate (1 – specificity).
Results
Demographic and Clinical Characteristics at Age 14
Reported in
Table 1 are the means of the variables used to match the 27 adolescents with and the 135 control subjects without psychotic-like experiences (no significant differences between the groups). Furthermore, the groups were not different on age or on alcohol, cigarette, and cannabis use in the previous year.
Task Activation Differences Between Groups
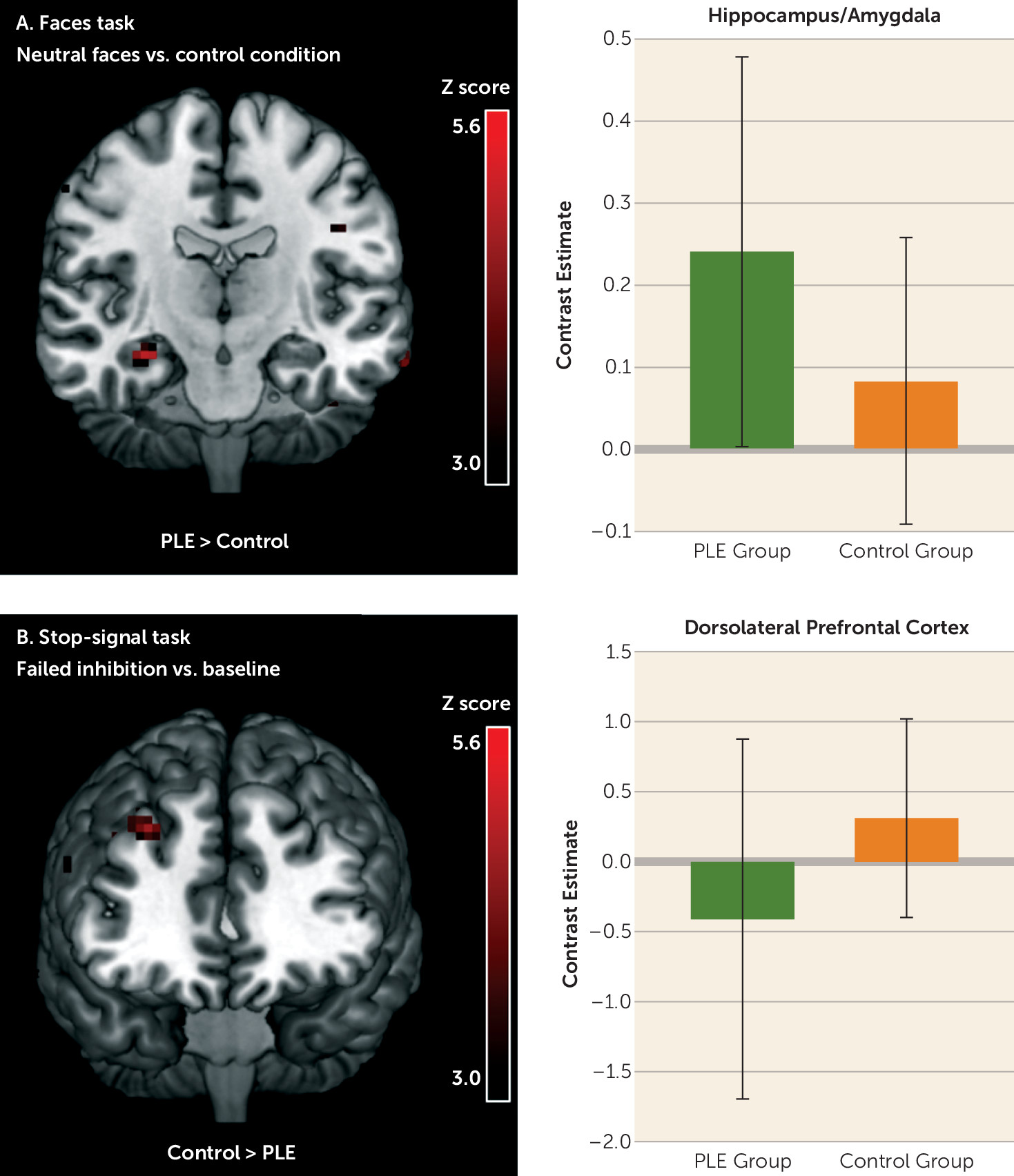
Between-group differences were present in small clusters in the three tasks (
Table 2). Only two significant clusters of activity differences survived the cluster-corrected threshold of 24 contiguous voxels: a hyperactivation of the right anterior hippocampus/amygdala during passive viewing of neutral/ambiguous faces and a reduced activity in the right DLPFC during failure to inhibit a motor response in youths with psychotic-like experiences (
Figure 1).
Prediction of Psychotic-Related Symptoms at Age 16
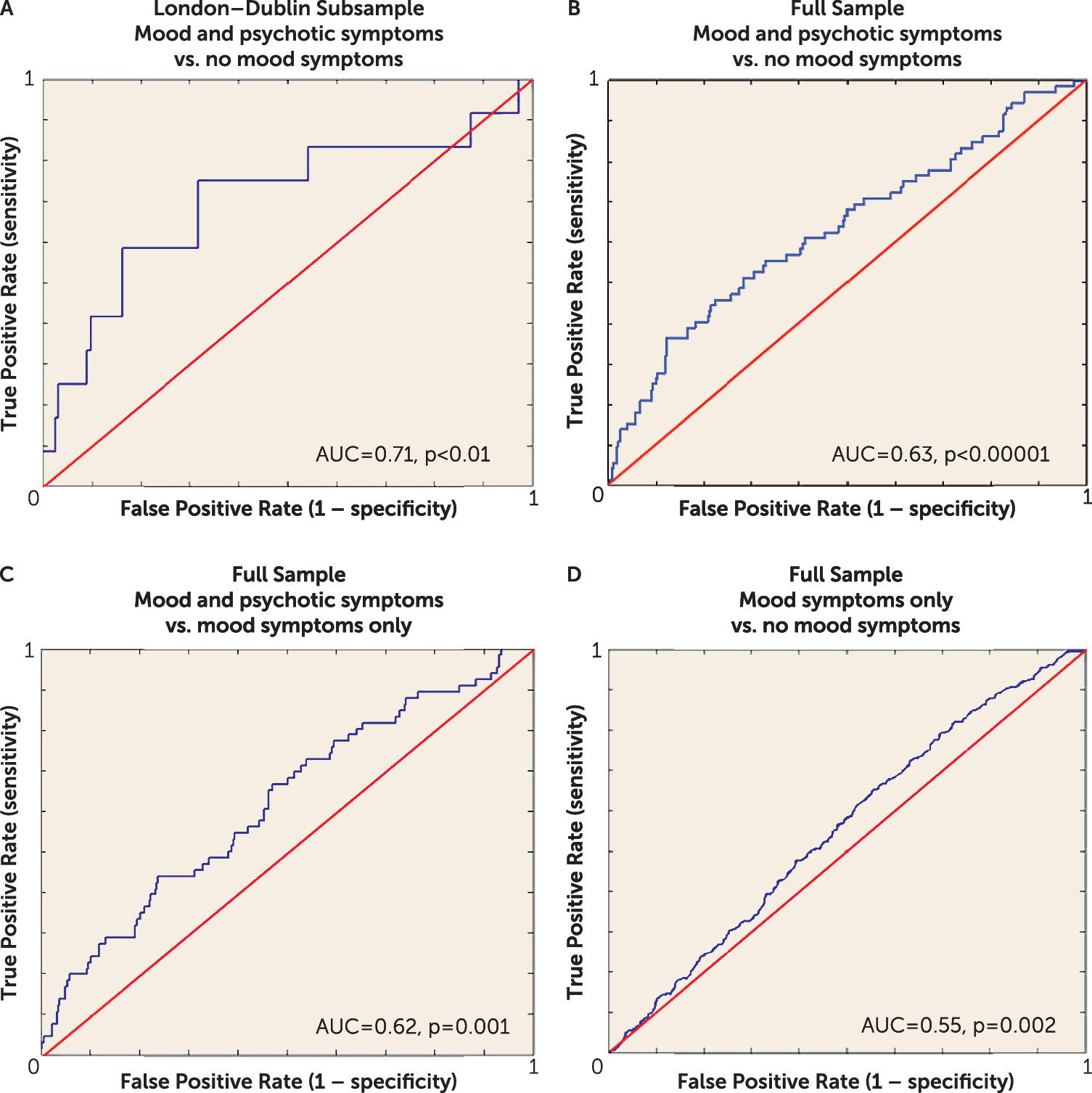
First, from the London-Dublin subsample, we differentiated youths reporting both mood- and psychotic-related symptoms at 16 (N=12) from those reporting no mood symptoms (N=154). The final model returned from this analysis had a mean AUC of 0.709 (95% CI=0.706–0.713, p<0.01) (
Figure 2A). This model included all brain regions that survived the more liberal threshold of 10 contiguous voxels (all regions reported in
Table 2) and controlled for psychotic-like experiences score at age 14 as well as demographic information (i.e., age, sex, handedness, and site). All features were present in at least 9 folds (out of 10) of the final model. In addition to psychotic-like experiences, the most robust brain classifiers were cerebellum activity during processing of angry faces and the hippocampus/amygdala activity during neutral faces processing (
Table 3, model A). The performance of each domain on its own (i.e., brain activity versus psychotic-like experiences) is displayed in Figure S1 in the
online data supplement. We could not significantly distinguish youths with mood symptoms only (N=80) from the other two groups, i.e., the group with both mood and psychotic symptoms (AUC=0.532, 95% CI=0.525–0.539, p=0.36) and the group with no mood symptoms (AUC=0.453, 95% CI=0.450–0.456, p=0.90).
In the second set of prediction analyses, using the full IMAGEN sample, we differentiated youths reporting both mood- and psychotic-related symptoms at age 16 (N=72) from those reporting no mood symptoms (N=673) and from those with mood symptoms only (N=451). The final models returned from this analysis had mean AUC values of 0.633 (95% CI=0.630–0.636, p<0.0001) and 0.615 (95% CI=0.614–0.617, p=0.001), respectively (
Figure 2, models B and C). These models included all brain regions that survived the more liberal threshold of 10 contiguous voxels and controlled for internalizing and externalizing behaviors, use of cigarettes, alcohol, and cannabis, as well as demographic information (i.e., puberty development index, handedness, age, sex, and site). In the classification of the mood and psychotic symptoms group relative to the no mood symptoms group, only internalizing and externalizing behaviors, cigarette and cannabis use, hippocampus/amygdala and cerebellum activity during neutral faces processing, and cerebellum activity during angry faces processing were present in at least 9 folds of the final model (
Table 3, model B). However, when classifying the group with mood and psychotic symptoms relative to the group with mood symptoms only, we found that all features were present in at least 9 folds of the final model, with cerebellum activity during angry faces processing, fusiform activity during anticipation of reward, internalizing behaviors, cigarette and cannabis use, and hippocampus/amygdala activity during neutral face processing making the strongest contributions to group classification (
Table 3, model C).
Finally, we differentiated individuals with mood symptoms only (N=451) from those reporting no mood symptoms (N=673) with a mean AUC of 0.553 (95% CI=0.552–0.553, p=0.002), barely better than chance (
Figure 2D). All features except the DLPFC activity during failed response inhibition were present in at least 9 folds of the final model. The most important classifiers were internalizing and externalizing behaviors, cannabis use, reduced activity from the cerebellum during neutral faces processing, puberty development scale, and site (
Table 3, model D).
Discussion
At age 14, across the brain networks implicated in emotion processing, response inhibition, and reward anticipation, the cluster-corrected markers of psychotic-like experiences included an increased response from the hippocampus/amygdala during processing of neutral material as well as reduced activity from the DLPFC during failed inhibition. Of note, hyperactivity of the hippocampus/amygdala during the processing of neutral faces further discriminated individuals with mood- and psychotic-related symptoms at 2-year follow-up relative to the other groups in both the London-Dublin subsample and the full IMAGEN sample, even when controlling for baseline psychotic-like experiences as well as cannabis and cigarette use. The cross-validation models best discriminated the group with mood and psychotic symptoms from the group with no mood symptoms, in comparison to discriminating the group with mood symptoms only from the group with no mood symptoms.
One of the most replicated neural markers of psychosis and clinical high-risk states is hypofunctioning of the PFC and DLPFC during executive functioning (
32). Our results support findings from other community-based studies of youths reporting psychosis-spectrum symptoms showing reduced PFC activity during working memory and response inhibition tasks (
9,
23). However, the activity of the DLPFC during the stop-signal task was a weak brain classifier for adolescents reporting both mood and psychotic symptoms relative to the other groups. A possible explanation might be that reduced DLPFC activation is directly related not to positive or mood symptoms, but more to disorganized symptoms or cognitive deficits (which were not assessed by our screening tools) (
9,
33). Consequently, DLPFC alterations would appear to be a promising neurofunctional marker of the clinical risk for psychosis when, in addition to positive and negative symptoms, significant cognitive impairments are observed, but not a robust marker in youth reporting psychotic-like experiences prior to a cognitive decline. It is worth mentioning that the use of a working memory task instead of response inhibition could have yielded more significant DLPFC results because working memory paradigms, in comparison to Stroop or Go-NoGo tasks, consistently elicit a more widespread locus of significant activation in the DLPFC and anterior cingulate cortex in both healthy control subjects and schizophrenia patients (
11).
The current exploratory study stresses the importance of an observed increased activity in the limbic network in the extended psychosis phenotype. Both fMRI and perfusion studies have highlighted increased hippocampal activity at rest and across cognitive tasks in clinically at-risk individuals (
32,
34). Interestingly, Schobel et al. (
35) demonstrated that baseline hypermetabolism of the hippocampus in clinical high-risk individuals is directly related to a subsequent volume loss (via a hyperglutamatergic state), thereby supporting the heightened hippocampus activity as a highly promising early marker of vulnerability to psychotic disorders. In the context of emotion processing, a recent meta-analysis showed that the apparent deficit in amygdala activity observed in individuals with a psychotic disorder during the viewing of negative material may be explained by an elevated amygdala response to neutral material (
15). These findings have led some authors to propose that abnormalities in salience attribution might be core to the extended psychosis phenotype, rather than stress reactivity per se (
36). Thus, the increased neural response to neutral information may reflect an atypical assignment of motivational salience to these stimuli (
37). Results from other cognitive studies showing an impaired decoding of facial expressions in patients with psychosis and high-risk populations further suggest that the abnormal neural activity in the current study might be due to an erroneous identification of neutral faces specifically. For instance, children and adolescents reporting psychotic-like experiences overattribute significance (i.e., negative valence) to neutral faces (
38). Since impaired emotion recognition is linked to declining social functioning in high-risk populations (
39), it represents a potential target for strategies to prevent psychosis symptoms in at-risk youth, prior to subsequent impaired social functioning.
Since cerebellar activity significantly contributed to the classification of youth with mood- and psychotic-related symptoms relative to the other groups, even in the absence of a marked difference in functional activity between individuals with and without psychotic-like experiences at age 14, its role in emotion processing in the psychosis spectrum remains elusive but deserves to be clarified in the future.
No cluster-corrected differences in brain activity between 14-year-olds with and without psychotic-like experiences were observed during reward anticipation. Even when a more liberal cluster threshold was used, significant activity related to reward anticipation did not robustly contribute to discriminate the groups at age 16. These findings are inconsistent with recent fMRI studies showing a blunted response from the ventral striatum during reward processing in psychosis and high-risk individuals (
18,
40). A possible explanation for this negative result may be given by the finding by Radua et al. (
18) of a negative correlation between striatal activity and the severity of negative symptoms in both patients and individuals at clinical risk for psychosis. Here, only positive experiences/symptoms were assessed.
Limitations
The use of an extended risk phenotype (i.e., youths self-reporting psychotic-like experiences) may constitute both a strength and weakness. While it might be too liberal to predict vulnerability to specific disorders, particularly those with very low prevalence, one advantage of this approach is that it might capture a dimension of vulnerability that is implicated in a number of different psychopathological outcomes. The current study also did not investigate interactions with family, substance misuse, and genetic data, which might further clarify how this extended phenotype is implicated in future psychiatric outcomes. Another potential limitation to the study is that the use of the bipolar module at age 16 may have underestimated the emergence of psychotic symptoms in the group with no mood symptoms. However, the prevalence of psychotic symptoms is low at the end of adolescence (e.g., 5%−7%) (
1). Finally, the timeframe for studying outcomes was relatively brief and might predate the typical age at onset of psychotic disorders; however, this might also be considered a strength, as we were able to detect relevant brain-related abnormalities before psychotic experiences begin to cause significant functional and cognitive impairment and substance misuse and require medical intervention.
Conclusions
The results of the present study suggest that an aberrant neural response to nonsalient stimuli may be an important early vulnerability marker for psychosis, at least in the context of mood fluctuations. These findings might help to guide early intervention strategies for at-risk youth. It has yet to be determined whether individual differences in emotional reactivity to nonsalient stimuli can be modified in young adolescents and whether such modifications have any clinical significance for high-risk youth.