Disruption of the inhibitory mechanism activated by S1 is postulated to reflect a fundamental neural deficit in schizophrenia that contributes to attentional difficulties associated with the illness (
3). Although P50 suppression deficits in schizophrenia appear to be robust, evidence relating this impairment to overt clinical symptoms and cognitive disturbances is mixed. Some studies demonstrate clear associations between P50 abnormalities and clinical symptom ratings (
4–
8), whereas other studies do not (
9–
12). In the domain of cognitive performance, P50 is associated with working memory and attention-related processes in schizophrenia (
5,
13,
14), although again, contradictory findings cast doubt on these effects (
15–
17 [for a review, see reference
16]). Although evidence linking P50 to symptoms and cognition may be spurious, the null findings could reflect underpowered studies or dichotomized scores on key variables, thereby reducing statistical power and underestimating effect estimates. Reliance on heterogeneous clinical composite measures that include domains presumed to be minimally related to attention (e.g., affective flattening, anhedonia) may also obscure important relationships between P50 suppression and specific symptoms (
9,
11).
Results
Demographic and clinical characteristics of the study participants are summarized in
Table 1. Because of group differences in age between schizophrenia patients and healthy comparison subjects, relevant analyses were repeated with age as a covariate. The covariate did not alter any significant results, and group differences without age as a covariate are reported below. The two groups were matched for highest parental level of education. As might be expected, schizophrenia patients and healthy comparison subjects differed in their years of education, given the likely influence of illness on the level of education achieved. Group comparisons were also repeated with level of education included as a covariate, which again did not alter the results. Symptom levels were generally mild-to-moderate for schizophrenia patients.
P50 Suppression
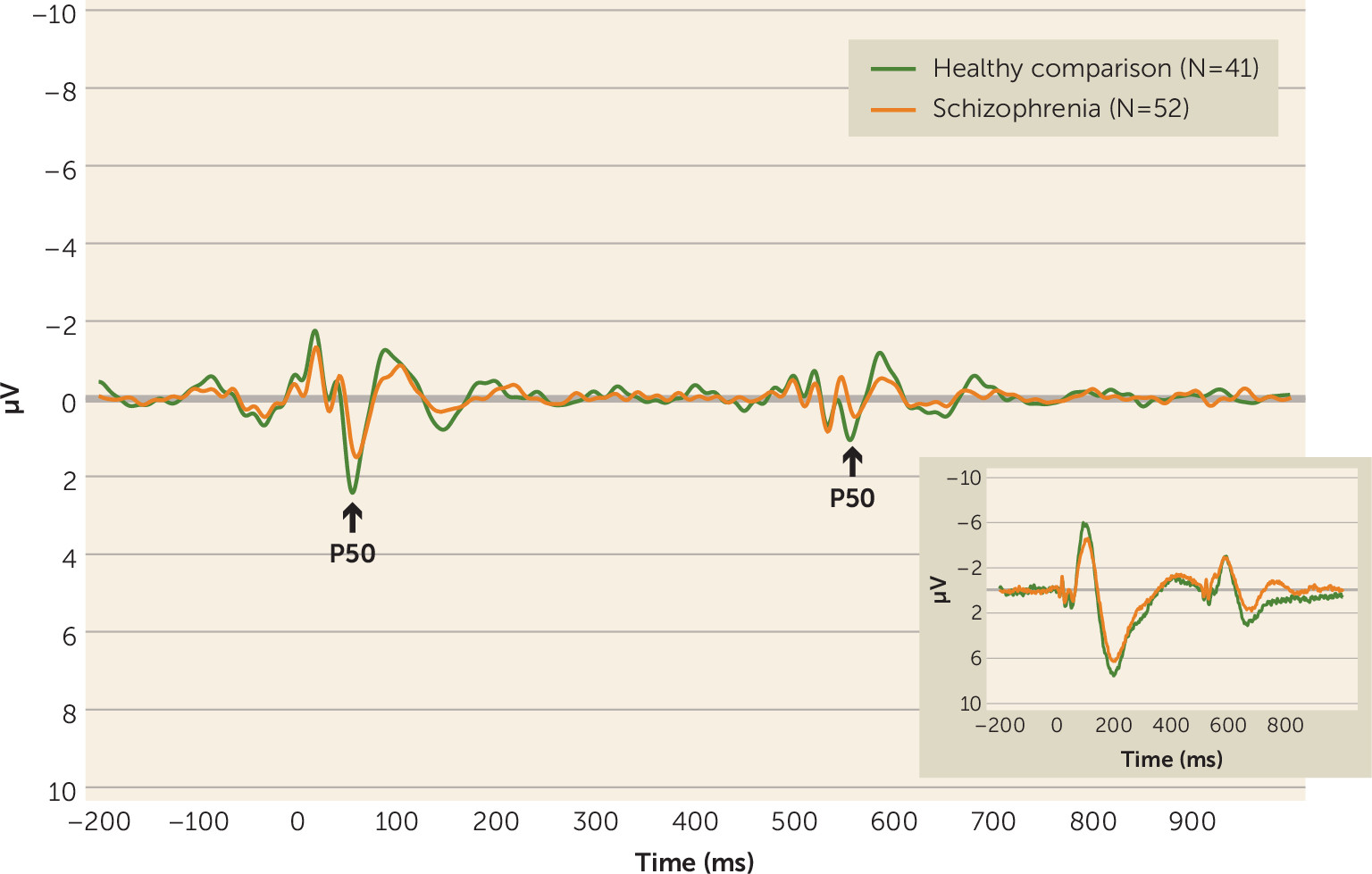
Grand-average event-related potential waveforms for each group are shown in
Figure 1. P50 mean amplitudes and suppression ratios are presented in
Table 1. Consistent with previous reports, P50 ratio scores indicated poorer suppression in schizophrenia patients compared with healthy comparison subjects (p=0.006) (
Table 1). A main effect of stimulus (F=125.65, df=1, 91, p<0.001; ηp
2=0.580) confirmed suppression and was qualified by a group-by-stimulus interaction (F=6.10, df=1, 91, p=0.015; ηp
2=0.063). Post hoc tests determined that P50 amplitude in response to S1 tended to be attenuated in schizophrenia patients compared with healthy comparison subjects (F=2.93, df=1, 91, p=0.090; ηp
2=0.031). Although the magnitude of the S2 amplitude response in the patient group was larger than that for the comparison group, the group difference was not statistically significant (p=0.612).
P50 and Clinical Symptoms
As shown in
Table 2, a significant positive association was observed among schizophrenia patients between P50 ratio scores and the SANS summary score but not the SAPS summary score. Correlations with each SANS subscale measure indicated that the association with P50 suppression was evident for only the global inattention subscale. Consistent with an inhibitory gating deficit, this effect was restricted to P50 amplitude in response to S2, such that the significant association between poorer suppression and clinical ratings of greater attentional impairment (r=0.338, p=0.014) did not extend to P50 in response to S1 (r=0.064, p=0.654).
To address the specificity of the association between P50 suppression and clinical ratings of attentional difficulties in schizophrenia patients, a hierarchical linear regression assessed the contribution of the inattention score after accounting for all other SANS subscale scores. This analysis revealed that inattention (β=0.394, p=0.009) uniquely explained 12.8% of the variance in P50 ratio scores, above and beyond the 7.2% of the variation accounted for by the other SANS subscale scores.
P50 and Cognitive Performance
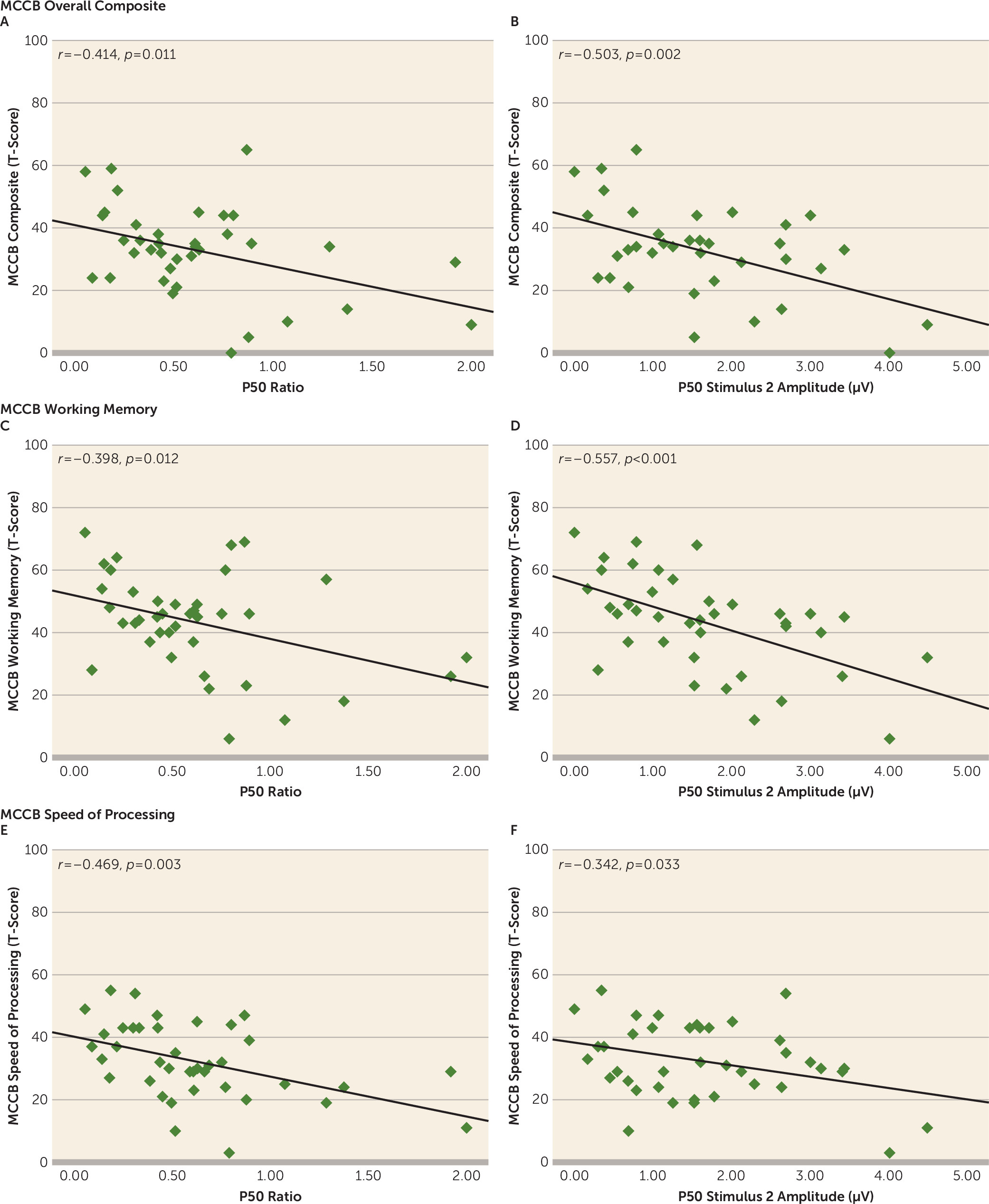
The MATRICS Consensus Cognitive Battery performance results are summarized in
Table 1. Zero-order correlations between P50 measures and cognitive variables of the consensus battery are presented in
Table 3 for the 39 schizophrenia patients who underwent cognitive testing. P50 ratios correlated with overall composite scores on the battery, indicating that impaired suppression was associated with poorer cognitive performance. As predicted, this effect was evident in significant associations with speed of processing and working memory performance (
Figure 2). Specifically, poorer P50 suppression was related to impaired performance during both verbal (r=–0.330, p=0.040) and visual (r=–0.381, p=0.017) working memory tasks. There were no statistically significant associations between the P50 ratio score and the other consensus battery domains (
Table 3).
An examination of the relative contributions of S1 and S2 amplitudes to the relationship of P50 ratios and cognitive performance revealed that a significant correlation with P50 in response to S2 drove this relationship for working memory (r=–0.557, p<0.001) and speed of processing (r=–0.342, p=0.033) performance, as well as for the consensus battery overall composite score (r=–0.503, p=0.002). There were no significant associations involving P50 amplitude in response to S1 (working memory: r=–0.160, p=0.331; speed of processing: r=0.249, p=0.126; overall composite: r=–0.056, p=0.743).
Effects of Medication, Smoking, and Symptom Severity
When analyses were repeated and covaried for chlorpromazine-equivalent dosages of antipsychotic medications and the number of cigarettes smoked during the past week, all reported significant results remained statistically significant. Similarly, all symptom relationships remained statistically significant when the sample was restricted to the 45 patients receiving risperidone. Furthermore, symptom severity did not account for any of the observed relationships between P50 and cognitive performance, since all associations with clinical and cognitive variables remained significant when adjusting statistically for SAPS and SANS global scores (all p values <0.05).
Discussion
This study investigated linkages between clinical symptoms, cognitive dysfunction, and pathophysiological mechanisms associated with auditory sensory processing deficits in schizophrenia. Findings confirmed the hypothesis that P50 suppression abnormalities are associated with such core clinical characteristics of schizophrenia as symptoms of inattentiveness, thereby clarifying the clinical significance of P50 suppression deficits. Although analogous results involving clinician-rated attentional impairment measures have been described previously (
5,
8), the present results are based on a larger patient sample, and a more powerful statistical approach was achieved by retaining the continuous range of information offered by SANS and P50 ratio scores. The results also underscore the specificity of the association between impaired P50 inhibitory processing and clinician-rated inattention in schizophrenia, since other negative symptom domains were not similarly implicated.
Beyond relating P50 suppression to a clinically observed core feature of schizophrenia, results from the present study link auditory sensory dysfunction to impairments involving working memory and speed of information processing. These findings corroborate the findings of previous studies (
5,
13,
14,
16) and extend such work by use of standardized measures from the MATRICS Consensus Cognitive Battery, a cognitive test battery on which schizophrenia patients reliably show compromised performance (
18). However, these findings contrast with those of one study in which P50 ratio scores were unrelated to cognitive performance in similar domains among medicated patients with chronic schizophrenia (
17). Illness duration and prolonged exposure to antipsychotic medications may account for this discrepancy.
The pattern of findings observed in this study is substantiated by research on the neural mechanisms of P50 sensory impairments. Source localization studies implicate a neural network involving the prefrontal cortex—particularly the dorsolateral prefrontal cortex—the superior temporal gyrus, the hippocampus, and the thalamus in the generation and suppression of P50 (
26,
27). Notably, the dorsolateral prefrontal cortex and hippocampus have been associated with working memory dysfunction in schizophrenia (
28,
29), while prefrontal cortex, hippocampus, and superior temporal gyrus structural and functional abnormalities have been implicated in efficient information processing (
30,
31). Furthermore, our results parallel a recent study, conducted by Tregellas et al. (
29), which found that hippocampal dysfunction was associated with poorer working memory performance on the MATRICS Consensus Cognitive Battery, consistent with the suggestion that disrupted hippocampal activity contributes to impaired sensory information processing. Taken together, P50 suppression deficits appear to involve impaired interactions between frontal and temporal brain regions, and these early sensory processing difficulties may, in turn, contribute to cognitive symptoms in schizophrenia.
Another important consideration is the complex relationship between working memory and attention, which have been linked behaviorally and neurobiologically (
32). Consistent with our findings of an association between working memory and enhanced P50 suppression, individuals with high working memory capacity may be more successful in resisting attentional capture by salient but irrelevant stimuli than those with lower capacity (
33). Optimal performance on working memory tasks utilized in the present investigation may also rely on attentional processes that influence maintenance of the material to be remembered (
34). Likewise, tasks measuring attention often include working memory demands to varying degrees (
35), and clinically rated inattention is likely to involve temporary disruptions in working memory. Furthermore, impairments on purported tests of processing speed (e.g., coding tasks) appear to be attributable in part to attention and working memory deficits, since efficient performance requires the ability to rapidly bind representations in working memory to improve accurate speeded performance (
36). Regardless, present results provide evidence for an important link between deficient P50 suppression and cognitive performance deficits in schizophrenia.
The absence of an association between P50 suppression and performance on the attention/vigilance task in this study highlights the possibility that P50 is related more closely to efficient information processing and working memory than to short-term, focused, sustained attention and vigilance as assessed, for example, by the Continuous Performance Test-Identical Pairs version. Specifically, P50 suppression may be more critical to tasks that involve rapid encoding, brief maintenance, and manipulation of stimuli than to tasks that require ongoing monitoring and sustained attention. Similarly, the lack of an association between P50 and performance on the MATRICS Consensus Cognitive Battery attention/vigilance task is not necessarily inconsistent with our finding of a relationship with inattentiveness on the SANS. Given that this global clinical rating is based on inattentiveness during social behavior and mental status testing, it may be that the construct better captures aspects of attention associated with speeded information processing and working memory rather than sustained attention and vigilance. This possibility is consistent with findings suggesting an absence of a relationship between P50 suppression and performance on the degraded stimulus Continuous Performance Test (
17). In fact, the apparent divergence from studies reporting positive associations (
5,
13) might be attributable to their reliance on tasks (i.e., digit vigilance and the Gordon Diagnostic System Continuous Performance Test) eliciting dimensions of attention other than those examined in this study (e.g., selective attention, vigilance, or orienting/shifting) or involving other task parameters (e.g., provision of feedback, time restrictions).
It is noteworthy that in addition to the P50 ratio score, our primary findings involved relationships between cognitive dysfunction and P50 S2 amplitude but not with the P50 S1 response. This pattern of findings is consistent with a dominant model of P50 sensory gating, whereby S1 activates inhibitory neuronal mechanisms that suppress or inhibit the response to the identical S2, resulting in attenuation of P50 in response to S2 (
3). Findings regarding the relative importance of a P50 S1-amplitude deficit have been debated in the literature. A meta-analysis demonstrated substantial heterogeneity in S1-amplitude group differences across studies and suggested that S1 amplitude alone does not differentiate schizophrenia patients from healthy subjects as reliably as the P50 ratio (
2). The present results support the possibility that patients with schizophrenia, and particularly those with greater working memory deficits, have insufficient activation of P50 inhibitory mechanisms.
The present research did not include medication-free patients, since at the time of participation, all patients were prescribed antipsychotic medications. Antipsychotic medications may affect some cognitive processes (
37), although findings have been mixed (
38). With respect to P50, neither first-generation nor second-generation antipsychotic medications—with the possible exception of clozapine (
39) and, to a lesser extent, risperidone (
8)—have been shown to improve sensory processing deficits in schizophrenia. Statistically accounting for chlorpromazine-equivalent dosages and limiting the study sample to recent-onset patients who were stabilized on risperidone did not modify the results. Therefore, the systematic impact of antipsychotic medications on our study findings was likely minimal. Additionally, although participants were assessed for substance abuse and/or dependence with the SCID, the study is limited by the lack of urine toxicology screens to verify abstinence prior to EEG assessment.
Taken together, results of this study implicate P50 inhibitory processing deficits with core features of schizophrenia. Furthermore, our findings substantiate P50 as a promising indicator of early sensory processing abnormalities by confirming the presence of inhibitory P50 deficits in schizophrenia and by demonstrating their associations with clinically rated inattention and working memory and processing speed performance. These results are consistent with the RDoC initiative and provide evidence that P50 suppression is a viable and promising manifestation of pathological mechanisms that can be used as targets for interventions that aim to improve cognitive impairments in schizophrenia (
40). Indeed, there is encouraging evidence to suggest that inhibitory gating deficits in individuals with schizophrenia are modifiable by cognitive training (e.g., see reference
41). Further research will help to clarify whether P50 suppression deficits are amenable to other forms of interventions while also determining whether such benefits extend to other aspects of cognition.