The incidence of depression rises markedly in adolescence (
2), potentially because of maturing reward-system function (
3). Most evidence linking aberrant reward processing to adolescent depression derives from task-based functional MRI (fMRI) studies targeting reward-related areas, such as the striatum (
4,
5). However, few studies have adopted network-based approaches, and most are cross-sectional (
5). Given the distributed nature of neural perturbations in depression, research is needed applying network-based approaches to intrinsic functional connectivity (iFC) data (
6–
8). Such work quantifies the degree to which brain “nodes” (
9) facilitate signal integration among network components. Applying this approach to longitudinal data could support inferences about causality (
5). While iFC studies implicate reward-network function in depression (
10,
11), most studies have examined small, clinically referred samples of adults on medication. There is a particular need for longitudinal studies of iFC in adolescent depression, to extend promising cross-sectional results (
12).
We used a longitudinal design to link reward-network iFC to later risk for a depressive disorder in a community-based adolescent sample. Based on previous findings, we hypothesized that aberrant ventral striatal iFC in early adolescence increases risk for future depressive disorder at 3-year follow-up (
4). Previous work suggests that such aberrancies reflect perturbed striatal integration of coalescing signals from a distributed reward network encompassing the ventral tegmental area, the anterior cingulate cortex, and the ventromedial prefrontal cortex (
13–
15). We quantified this integrative function through a measure of ventral striatum “node strength” (i.e., degree centrality), assessed as the region’s weighted sum of connection with other reward-network regions. We stringently tested this hypothesis by probing the existence of a putative reward network (
10) in separate discovery and replication samples, both assessed with relatively conservative statistical thresholds. We then assessed specificity by 1) evaluating other reward-system-related brain areas in the prediction of depression and 2) testing striatal node strength as a predictor of other psychiatric outcomes, including anxiety disorders, attention deficit hyperactivity disorder (ADHD), and substance abuse.
Discussion
Using a community-based sample, we found that ventral striatal iFC predicted new-onset depressive disorder 3 years later. We also found evidence of specificity: connectivity of the striatum, but not other regions, predicted depressive disorder, and striatal connectivity predicted depressive disorder, but not other psychopathology. The results of our longitudinal design provide novel evidence for the involvement of the reward network in the pathogenesis of depression.
Several clinical and basic science considerations implicate reward processing in depression. From a clinical perspective, there is the long-standing observation that a subset of depressive behaviors, such as reduced energy and motivation, are related to changes in reinforcement schedules (
28,
29). These notions underpin therapeutic approaches, in particular behavioral activation, a key component of cognitive and behavioral therapy in depression (
30). These observations converge with basic science findings about reward processing. Several experiments demonstrate the key role that dopaminergic signaling in ventral striatal areas plays in reward valuation and effort expended toward reward (
31,
32). In recent years, fMRI has enabled scientists to probe activity in deep brain areas such as the ventral striatum and thereby provide crucial links between long-standing clinical notions and basic science. Indeed, there is mounting evidence from reward task–based fMRI that reduced activity in the striatum is important in the etiology of depression (
3,
5,
33). However, there is a need to understand the distributed brain patterns of perturbations in depression (
6), and network measures using iFC are well suited to this approach. Unlike task-based fMRI, iFC does not rely on a behavioral paradigm and is therefore less confounded by issues such as ability or motivation to engage with a task (
34). This is particularly important when studying developmental effects, where standardizing a task across different age groups can present a daunting challenge. Therefore, since both reward processing and depression prevalence vary with development (
2,
35,
36), iFC appears well suited to their study.
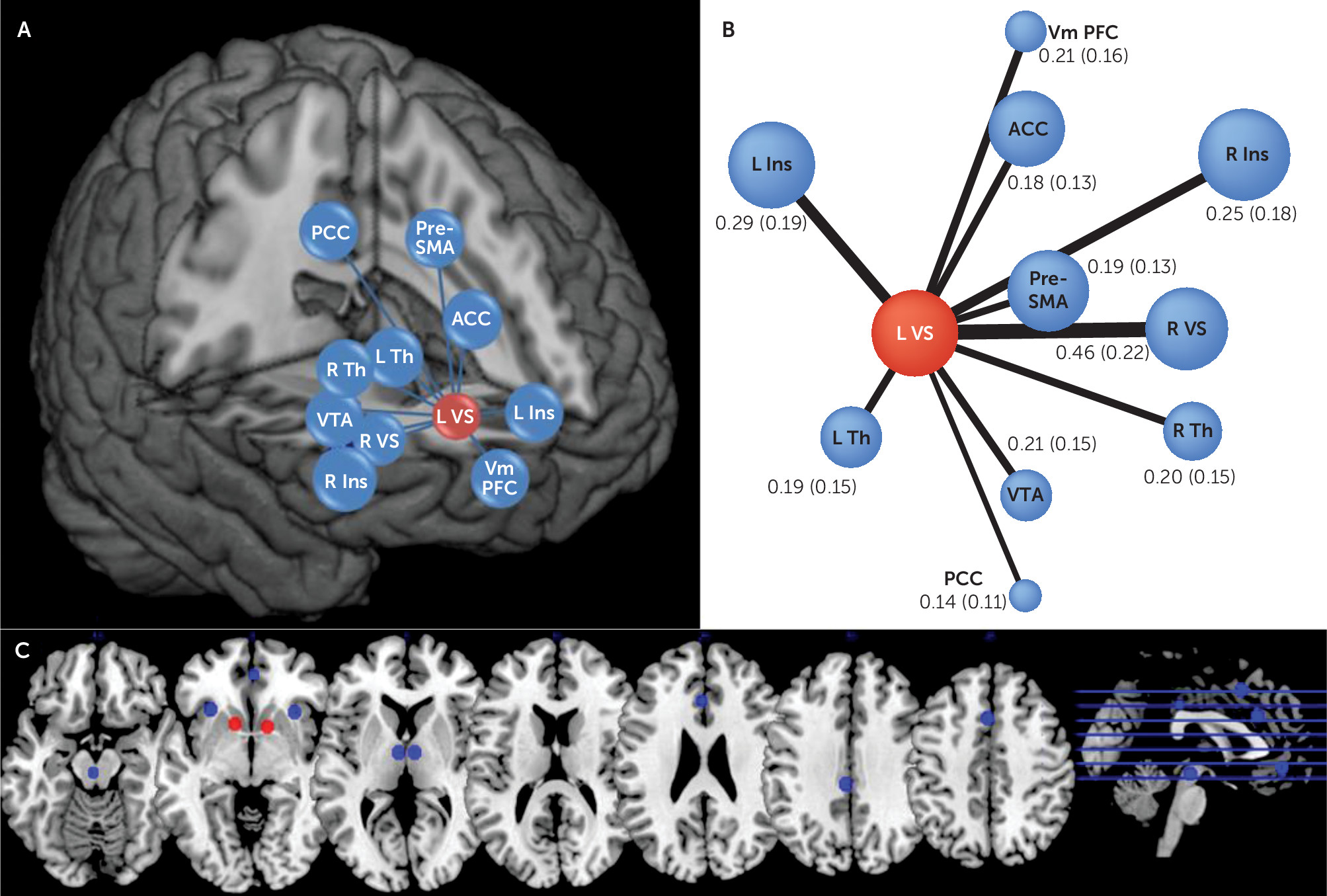
Probing the connectivity of a reward network allowed us to show that increased left ventral striatum node strength predicts depressive disorder at follow-up. Our first step was to show resting-state coupling between brain regions typically activated during reward-related behaviors (
3,
26,
37). Then, we computed node strength—an important network measure that captures the centrality of a given node within a network (
9). The left striatum was the only node whose strength predicted depressive disorder. This suggests that the left ventral striatum is integrating information from various areas of the reward network, including those previously implicated in adolescent depression, such as the anterior cingulate cortex (
13,
14). Having rigorously probed ventral striatal iFC within the reward network, we then used it in the prediction of depressive disorder 3 years later. We demonstrate that left ventral striatum node strength predicts new-onset depressive disorder (that is, after excluding depressive disorder cases at baseline).
This finding indicates that perturbed connectivity in the reward network is not merely a consequence of experiencing depression, but predates the expression of the disorder. Thus, striatal iFC is a marker of depressive disorder risk and supports its role in the pathogenesis of depression, although our observational study cannot offer conclusive evidence about its causal role (i.e., striatal iFC could still be an early marker but not be itself implicated in the illness). It should be noted that we did not find a significant association between striatal node strength and depressive disorder at baseline. However, the low prevalence of depressive disorder at baseline, which is expected given the young age of participants at that point, may have diminished statistical power to demonstrate this association.
Our study finds that increased rather than decreased iFC predicts depressive disorder. One possible explanation for this finding is that increased iFC is an attempt at compensating for the blunted striatal response to rewards that has been described in depression (
6). Coupling resting state connectivity studies with functional imaging probing the ventral striatum could help test this hypothesis. Alternatively, hyperconnectivity within the reward network could reflect a primary pathogenic process in its own right. Resembling hyperconnectivity found within other networks in depression studies, such as the default mode network (
7), increased iFC may itself impede adequate reward processing during reward-related tasks, leading to blunted ventral striatum signals, a hypothesis that could also be tested in longitudinal studies that employ serial resting-state and task-based fMRI studies. A previous study found decreased iFC within the reward network (
10), yet this discrepancy could be explained by the fact that these were adults who already had depression and, unlike our adolescents, were on medication.
Lastly, since psychiatric disorders in youth are frequently comorbid (
38) and ventral striatal dysfunction is implicated in other disorders (
39,
40), we examined whether left ventral striatum node strength could predict diagnoses other than depressive disorder. Supporting the specificity of our main result, the node strength of the left ventral striatum did not significantly predict anxiety disorders and ADHD. We also investigated the association of the left ventral striatum node with another reward-related phenotype, any substance use. One previous longitudinal study (
40) showed that stronger cortico-striatal iFC (right dorsolateral prefrontal cortex, dorsomedial prefrontal cortex, and pre–supplementary motor area) predicted earlier onset of alcohol and substance use. We did not find this association. However, our sample was younger than the expected age for onset of substance use. Further evidence that this is a limitation for detecting substance use findings is that age was positively associated with any substance use (see Table S5 in the
data supplement). Future work dissecting the various anatomical and functional components of reward processing may identify differential predictions between major depression and other disorders, such as substance-related and addictive disorders.
Our study has a number of strengths. Our discovery and replication analysis addresses recent concerns regarding the lack of replicability in neuroimaging studies (
25). Furthermore, adolescent major depressive disorder studies typically rely on cross-sectional designs and relatively small clinically referred samples, whereas ours was a longitudinal study in a large community-based sample of unmedicated adolescents. However, our study also has limitations. We investigated a specific brain network, despite evidence of several networks being implicated in both major depression and typical development (
6,
7). The main advantage of this approach, which narrows brain regions based on previous data, is to avoid spurious associations found in whole-brain investigations. In addition, there was attrition at follow-up, which can introduce bias. However, our loss at follow-up was relatively modest (10% of the imaging sample). Also, adolescence and young adulthood are the age of maximum incidence of depression. This may have led to an underestimation of the strength of our effects, since most participants in our study were in their early adolescence. Moreover, we did not collect child reports of depression at baseline because of the low reliability of youth report in early childhood (
18,
20). Finally, iFC data are sensitive to head motion (
22,
23). We addressed this issue using distinct techniques and rigorous thresholding. Our main results persisted after we imposed restrictive head movement parameters, which increases confidence in our findings.
In summary, we investigated the iFC of the ventral striatum within the reward network in a community sample of adolescents. Increased ventral striatum node strength was associated with an increase in odds of depressive disorder by approximately 50% after 3 years. This underscores the importance of the brain’s reward network in the pathogenesis of depression and calls for further studies to make clinical use of these findings.