The United States is confronting an unprecedented epidemic of opioid overdose deaths. In 2015, unintentional overdoses claimed 52,404 U.S. lives, and nearly two-thirds (63.1%) of these deaths involved opioids (
1). Between 2000 and 2014, the annual rate of overdose deaths involving opioids tripled from 3.0 to 9.0 per 100,000 persons (
2). In addition to overdose deaths, there were more than 360,000 emergency department visits in 2011 related to nonmedical use of prescription opioids, which is more than double the number in 2005 (
3). In response to the increase in opioid-related morbidity and mortality, federal, state, and local governments are implementing a range of policies and programs aimed at reducing inappropriate prescribing of opioid analgesics (
4–
6) and increasing access to treatments for opioid dependence (
7–
9).
Because the national increase in opioid-related deaths has coincided with escalating use of prescription opioids, concern has focused on the contribution that therapeutic opioids to treat chronic pain may make to opioid-related mortality (
12–
14). In a case-control study, opioid-related decedents were three times as likely as the comparison group to have had a chronic pain condition (
15). These findings raise the possibility that people with chronic pain who die of opioid-related conditions may be a distinct subgroup of opioid-related deaths, though to our knowledge, no previous research has examined this issue.
Method
Sources of Data
Opioid-related deaths were extracted from 2001–2007 national (45 states, not including Arizona, Delaware, Nevada, Oregon, and Rhode Island) Medicaid Analytic Extract data from the Centers for Medicare and Medicaid Services. Dates and cause of death information of the study decedents were derived from linkage to the National Death Index, which provides a complete accounting of state-recorded deaths in the United States and is the most complete resource for tracing mortality in national samples (
17). The National Death Index segment variables were linked to the Medicaid administrative data by the National Center for Health Statistics. The project was reviewed and approved by the Centers for Medicare and Medicaid Services and the Rutgers University Institutional Review Board.
Study Samples and Follow-Back
Because in adults ages ≥65 years Medicare-covered services render Medicaid claims records incomplete, we restricted the sample to individuals who were ≤64 years of age at the time of opioid overdose death. Following the Centers for Disease Control and Prevention (CDC) definition of drug overdose deaths involving opioids, deaths included poisonings by and adverse effects of opioids (T40.0X), heroin (T40.1X), other natural and semisynthetic opioids (T40.2X), methadone (T40.3X), synthetic opioids other than methadone (T40.4X), and unspecified narcotics (T40.6X) (
1). In order to assess service use during the 12 months preceding death, we further restricted the sample to decedents who were continuously enrolled in the Medicaid program for at least 12 months prior to death.
Opioid-related deaths were partitioned by occurrence of at least one inpatient or at least two outpatient claims with a chronic noncancer pain diagnosis in the last year of life (
18) (see Table S1 in the
data supplement accompanying the online version of this article). To increase the generalizability of the results, patients with cancer diagnoses were included in the study sample. However, neoplasm related pain (ICD-9-CM: 338.3) was not included in the definition of the chronic noncancer pain variable.
Demographic and Clinical Characteristics
Based on Medicaid eligibility data, decedents were classified by sex, age in years (<24, 25–34, 35–44, 45–54, 55–64), and race/ethnicity: Hispanic; white, non-Hispanic (white); black, non-Hispanic (black); and other, non-Hispanic (other), including American Indian/Alaskan Native, Asian, Native Hawaiian/Other Pacific Islander, and more than one race.
Service claims classified decedents by any outpatient visits and any visits with substance use, drug use, opioid use, and alcohol use disorder diagnoses during the 30 days and 12 months before death (see online Table S1). Because acute services separated by fewer than 2 days may represent single care episodes (
19), service dates for in-hospital deaths were initiated 2 days before the index inpatient admission.
Pharmacy claims classified decedents with respect to presence of filled prescriptions for opioids, benzodiazepines, their combination, antidepressants, antipsychotics, and mood stabilizers during the 30 days and 12 months before death. Decedents were also classified with respect to selected overdose (nonfatal poisoning) events during these time periods, including opioid, benzodiazepine, and other psychotropic medication overdoses (online Table S1).
Variables representing diagnosis of selected specific mental disorders on or within 365 days before the date of death were defined as drug use, opioid use, alcohol use, depression, anxiety, bipolar, schizophrenia, personality, and other mental disorders. At least one inpatient or two outpatient diagnoses defined clinical diagnosis of each mental disorder group. Decedents were also characterized with respect to selected causes of death (online Table S1).
Analysis
We compared the percentages of decedents in the two chronic pain diagnosis groups by the demographic, service use, prescription, and cause of death variables. In view of the large sample sizes and number of comparisons, two-tailed alpha was set at 0.001.
Discussion
Most persons with opioid-related fatalities were diagnosed with one or more chronic pain condition in the last year of life. Compared with people with opioid-related deaths without diagnosed chronic pain conditions, the decedents with chronic pain diagnoses were more likely to have also received diagnoses of substance use disorders and other mental health disorders. They were also more likely to have filled prescriptions for opioids, benzodiazepines, and other psychotropic medications and to have had a nonfatal drug overdose. The extent of health service use of this population prior to death, including clinical recognition of substance use and other mental disorders, may provide opportunities for detection of overdose risk and early intervention.
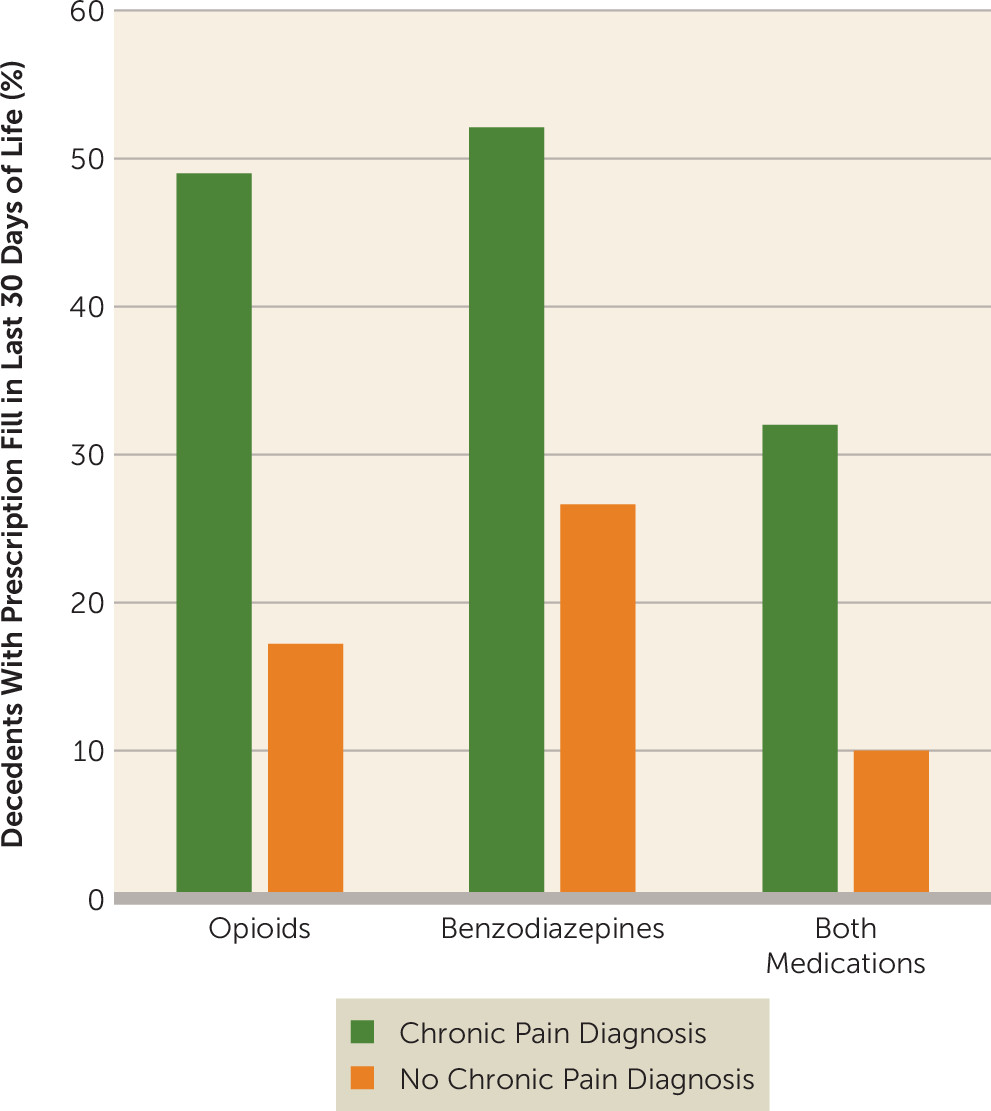
The prevalence of opioid prescriptions prior to fatal overdose greatly exceeded the corresponding prescription prevalence in the general population. In a 30-day period, approximately 8.8% of Americans fill prescriptions for analgesics (
20). By contrast, 36.8% of the opioid-related decedents filled opioid prescriptions in the last 30 days of their lives. In prior research, a roughly similar proportion (29.1%) of decedents in unintentional pharmaceutical overdose fatalities had received opioid prescriptions within 30 days of death (
10). Nearly half (49.0%) of decedents with clinically treated chronic pain received opioid prescriptions in the 30 days preceding death. This pattern raises the possibility that health care professionals may frequently be proximal sources of opioids in fatal overdoses. In evaluating these percentages, however, it is important to bear in mind that only a small proportion of Americans with even severe pain meet diagnostic criteria for opioid use disorders (0.75%) (
21), suggesting that most individuals with pain are at low risk for opioid overdose.
Benzodiazepines were also commonly prescribed to individuals prior to fatal opioid overdose. Among decedents whose deaths were related to opioids, 42.3% filled benzodiazepine prescriptions in the last month as compared with 4.6% of Americans who report taking anxiolytics, sedatives, or hypnotics in a 30-day period (
20). Decedents who had been diagnosed with chronic pain also frequently filled prescriptions for both benzodiazepines and opioids in the last month of life (23.5%). The safety threat posed by benzodiazepine and opioid prescription combinations arises from the potential for benzodiazepines to potentiate opioid-induced respiratory depression (
22). Most deaths involving opioids result from respiratory depression (
23). In roughly one in six opioid-related fatalities, benzodiazepine overdose contributed to the cause of death.
The public health risks of opioid and benzodiazepine coprescription are highlighted by recent increases in prevalence of such coprescription (
24) and a disproportionate increase in overdose deaths involving benzodiazepines with opioids (
25). Coordination of care across providers, routine use of prescription drug monitoring programs, and integration of these programs with electronic medical records might help reduce unintentional coprescription of benzodiazepines and opioids. Physicians might also reduce these safety risks by restricting benzodiazepine and opioid coprescribing to patients for whom alternative strategies have proven inadequate, closely monitoring for sedation and respiratory depression, and limiting such coprescription to the minimum clinically required dosage and duration (
26).
Approximately one in 20 (6.2%) opioid-related decedents had received medical care for a nonfatal opioid overdose in the last year of life. In a review of medical examiner records from West Virginia (N=295), approximately one in six (16.8%) decedents in pharmaceutical overdose fatalities had a lifetime history of nonfatal drug overdose (
10). To help place these percentages in context, the annual rate for medically treated opioid overdoses in the general population (California and Florida) was 50.5 per 100,000 population in 2010–2011, an approximately 300-fold difference (
27). The high relative proportion of opioid-related deaths that are preceded by nonfatal overdoses suggests that nonfatal opioid overdose is a strong risk factor for opioid-related death. This association supports assertive emergency department efforts to engage individuals in treatment for substance use disorders following nonfatal overdose. Yet, because the great majority of fatal opioid overdoses are not preceded by medically treated nonfatal overdoses, public health initiatives to reduce opioid-related deaths must extend beyond improving follow-up treatment for substance use disorder after nonfatal overdoses.
Many of the opioid-related decedents, especially those who had been diagnosed with chronic pain conditions, were also diagnosed with depression, anxiety, or other mental disorders in the last year of life. The high frequency with which clinical attention was devoted to mental disorders prior to opioid-related death, which was also reflected in the psychotropic medication prescribing patterns, suggests that these patients frequently presented complex clinical management challenges. Similar mental health service patterns have been reported in a Utah study in which friends and family reported on opioid-related overdose deaths (
28). Psychiatric disorders are common among individuals with chronic pain and opioid use disorders. In one analysis, nearly half of adults with co-occurring chronic pain and opioid use disorder met diagnostic criteria for a current anxiety (48%) or mood (48%) disorder (
29). Among adults receiving opioids for chronic pain, major depressive disorder and psychotropic medication prescriptions are also associated with an increased risk of prescription opioid dependence (
30). In the clinical management of chronic pain, a careful mental health history and periodic structured assessments may help detect opioid-related risks.
In the course of 1 year, less than one-quarter of adults with prescription opioid use disorders received any substance use treatment (
31). By comparison, a larger proportion of decedents in opioid fatalities (42.2%) were diagnosed with substance use disorders in the last year of life. However, only a small proportion of these diagnoses were opioid use disorder, and most decedents who received substance use disorder diagnoses during the last year did not appear to receive any substance use related services during the last 30 days. Dropout from treatment of substance use disorders may be common in the months preceding opioid-related deaths. Interventions that increase engagement and retention in treatment for substance use disorders could decrease the death toll of opioid overdoses.
This study has several potential limitations. First, there is a potential for misclassification of opioid-related overdoses, though our ICD-10 classification scheme is consistent with consensus recommendations from the Injury Surveillance Workgroup (
32) and the CDC (
1). Second, different results might have been obtained if privately insured and uninsured opioid-related fatal overdoses were included in the analysis, though Medicaid enrollees are a high-risk population (
16). Third, our study is based on data from 2001 to 2007. Opioid use, naloxone reversal, and service use patterns have changed since then in ways that may affect the reported service utilization patterns. For example, a recent marked increase in fentanyl-related overdose deaths has coincided with an increase in the supply of illicitly manufactured fentanyl and fentanyl analogs (
33). This and other recent trends in fatal opioid-related overdoses are not captured in the present study. Finally, we have no means of capturing opioids that were acquired from illicit sources or of measuring whether patients took medications, including opioids, as prescribed.
In the year prior to death, most individuals with opioid-related fatalities were diagnosed with chronic pain conditions, and many were diagnosed with psychiatric disorders and prescribed psychotropic medications. Although the claims histories suggest that clinical attention to substance use disorders was fairly common in the last year of life, few were diagnosed with opioid use disorder in the last 30 days of life. These service patterns highlight the need to improve the integration of specialized substance use services in pain treatment clinics and mental health centers.