Thyroid and mood disorders are both common and may co-occur in the same person, and psychiatrists must optimize treatment for patients with these comorbid disorders. In many cases, thyroid disorder is clear or preexisting. In other cases, thyroid dysfunction is not obvious and is suggested only by marginal abnormalities in thyroid biomarkers or poor response to standard antidepressant treatment. Optimal intervention requires knowledge of thyroid pathology and attention to the possibility of subtle thyroid dysfunction in patients with affective disorders.
The first case described above, from 35 years ago, alerted us to the possibility that some patients with normal thyroid test results might nonetheless have subtle thyroid dysfunction, enough to affect treatment for depression. Since then, we have treated many patients already receiving thyroid hormone supplementation for preexisting hypothyroidism, as well as patients without clear hypothyroidism, in whom, because of some evidence of thyroid dysfunction, we tried either levothyroxine (T4) or liothyronine (T3) to augment antidepressant treatment response. Most commonly, these patients had thyroid-stimulating hormone (TSH) levels in the high normal range and persistent depression, with low mood and low energy.
Thyroid Disorders and Monitoring of thyroid state
The thyroid gland produces the iodinated tyrosine-based hormones T
4 (thyroxine) and T
3 (triiodothyronine). Although more T
4 is released, T
4 is predominantly a prohormone. It is taken into cells by active transport and converted to T
3, which subsequently interacts with proteins that include nuclear receptors to modulate gene expression and numerous aspects of metabolism, including energy metabolism. Production of T
4 is determined by levels of TSH, released from the pituitary gland, in response to thyrotropin-releasing hormone (TRH), released from the hypothalamus. T
4 and T
3 are largely protein bound in serum, being carried by several proteins, including thyroid-binding globulin, transthyretin, and albumin (
1). The hypothalamus and pituitary monitor serum thyroid hormone levels, a process that provides a feedback mechanism for modulating TRH and TSH release and maintaining adequate amounts of thyroid hormone. Entry of thyroid hormone into the brain is mostly through capillary blood, although it also occurs through CSF (
1). As with serum carriers of thyroid hormone, there are numerous proteins that actively transport thyroid hormone into cells, metabolize them, and carry them to receptors (
2,
3).
Both excessive thyroid activity (hyperthyroidism) and inadequate thyroid hormone (hypothyroidism) are common, with hypothyroidism often associated with symptoms of depression (
4,
5). The most common causes of hypothyroidism are autoimmune disorders (Hashimoto’s thyroiditis) and inadequate thyroid hormone production due to surgical removal of thyroid adenomas. Both major depression and thyroid disease are two to three times as common in women as in men (
6), and there is evidence suggesting that hormone supplementation may be more effective in women (
7). In psychiatry, a common cause of hypothyroidism is lithium treatment, and thyroid hormone status is routinely monitored in patients receiving lithium. Smoking and race have minimal associations with thyroid function. Dopaminergic agents, glucocorticoids, and a few other medications can cause modest reductions in TSH release (
8). Antidepressants may be associated with small decreases in T
4 levels, although the meaning of that association, against a background of mild thyroid abnormalities in depression, is unclear (
9).
There are simple blood tests and good interventions to document and modulate thyroid state. However, it is important to note that while there are standard or normal laboratory result ranges, thyroid dysfunction, like many medical disorders, is a matter of degree, and the standard range cutoffs are guidelines (
10). For example, the normal high end of the TSH range is often quoted as 4.5 μIU/mL, but that does not necessarily mean that a patient is physiologically normal at 4.4 but abnormal at 4.6.
The management of overt thyroid disease is best handled by specialists. Evaluation involves multiple measures, including serum thyroid hormone levels (both free and bound levels), but the core of monitoring is of serum TSH levels (
8). This is because TSH is a reflection of adequate thyroid hormone in the periphery and the brain, as monitored in the pituitary and hypothalamus, respectively. The normal range of TSH has been the subject of ongoing debate, and some authorities, based on recent evidence, now define the upper limit of optimal TSH as being well below the long-quoted standard of 4–4.5 μIU/mL (
8,
11,
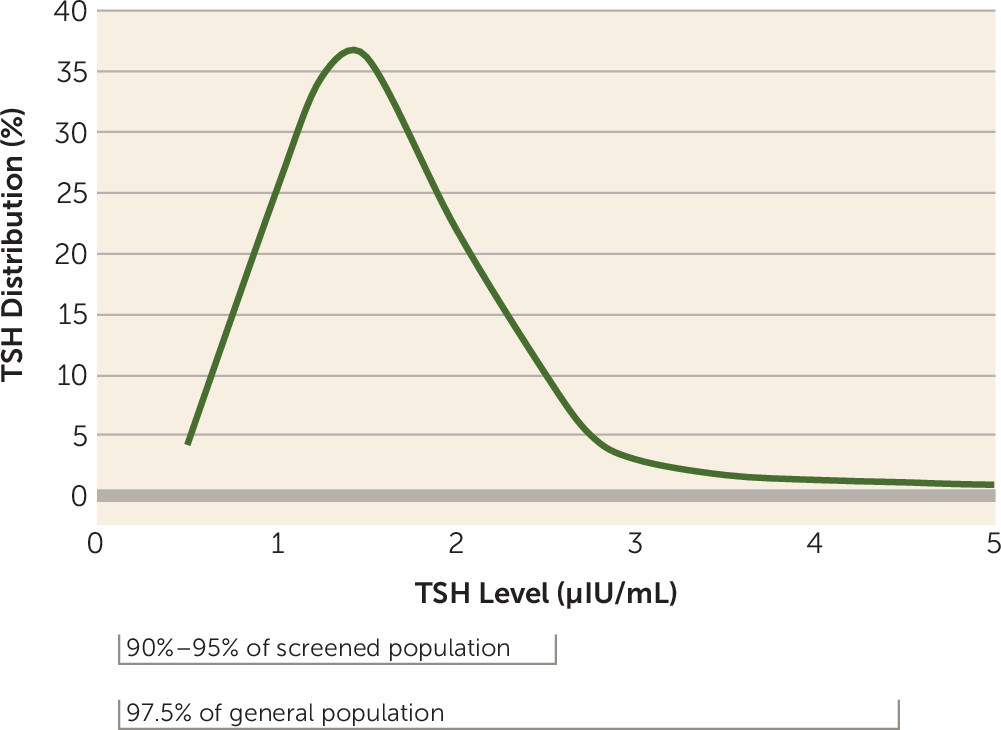
12). While most people (97.5%, the usual percentage included in a lab range) have TSH levels below 4 or 4.5, in subjects screened for thyroid health (and having no evidence of thyroid antibodies), some 90%−95% of people have TSH levels below 2.5 (
8,
11). In essence, the distribution of TSH levels in a healthy sample is not centered within the lab normal range (
Figure 1); it is skewed, with a large peak around 1.5 (1.43 for men and 1.67 for women) (
11). That is, most people have TSH levels toward the bottom of the TSH range (0.5–2.5), and there is a long “tail” of TSH values with a relatively small proportion of people with levels above 2.5 (
8,
11). Therefore, a TSH level above 2.5 is not physiologically optimal or normal for the vast majority of people. In fact, those with high-normal TSH levels compared with those with levels below 2.5 may actually have mild hypothyroidism, prodromal to overt hypothyroidism in ensuing years (
11,
13). These recent reassessments (
13) suggest that TSH levels above the peak may represent “occult thyroid disease,” and current reviews suggest that the target for TSH with supplementation should be below 2.5 (
14,
15) or even below 2 (
10).
The benefits of thyroid supplementation in people with minimal elevations of TSH level (above 2 or 2.5 μIU/mL) have not been fully explored. Studies of subclinical hypothyroidism (defined as a T
4 level in the normal range but a TSH level above 4–4.5 μIU/mL) are more common, and generally they have observed no short-term benefit of treatment in most patients with elevated TSH levels (
16). However, the studies to date include heterogeneous populations in which most study subjects have no or minimal complaints, including any symptoms of affective disorders. In addition, supplementation was conservative, with additional thyroid hormone given at very modest dosages, so that TSH level was often only minimally reduced (
17). Given the absence of complaints and such conservative treatment, benefits would not be expected. By comparison, several studies have suggested that there are short-term benefits from thyroid supplementation in patients with symptoms, often including complaints as simple as fatigue or low mood (
14,
18,
19). In addition, the current consensus is that treatment of subclinical hypothyroidism improves lipid profile and the long-term risk of stroke and cardiovascular disease (
14,
19).
Thyroid State and Mood Disorders
Thyroid hormones affect most aspects of cell development and function (
20,
21), so it is not surprising that there are documented relationships between thyroid state and psychiatric disorders. Specifically, thyroid disorders may increase the risk of mood disorders, and the rate of depression is modestly elevated in those with hypothyroidism (
4,
5). Also, when overt hypothyroidism is treated with thyroid supplements, symptoms of low mood often clear along with other features of thyroid hormone inadequacy (
22). However, despite the general effects of thyroid hormones on most body processes and despite some overlap in symptoms, such as changes in energy and appetite shared by thyroid and mood disorders, most patients with major depression show evidence of neither overt nor subclinical hypothyroidism (
23).
While depression is not strongly associated with overt thyroid disorder, it is associated with complex and subtle thyroid function irregularities, including modestly elevated TSH levels, modestly elevated T
4 levels, a blunted TSH response to TRH, and altered circadian release of thyroid hormone (
24). However, similar abnormalities of thyroid regulation have been observed in many illnesses as well as in response to stress (
7,
25), and many hormones and processes, not just thyroid function, show circadian abnormalities in major depression. Thus, these thyroid alterations may be nonspecific, not causally or crucially related to the pathophysiology of depression. Immune system abnormalities have been observed both in thyroid and depressive disorders (
26), but although the most common cause of thyroid disorders is an autoimmune disorder, dysregulated immune function, not autoimmunity, is more clearly documented in depression, as noted above. The relationships among immune, thyroid, and mood disorders remain matters for further research.
Overall, the evidence does not suggest that thyroid dysfunction underlies most depression or that substantial thyroid dysfunction is present in typical patients with major depression. Nonetheless, even a TSH level in the upper quartile of normal has been reported to be associated with a higher frequency of depressive episodes, more severe symptoms, and poorer response to treatment in people with major depression (
27). Pae et al. (
28) reported less severe but more treatment-resistant depression in euthyroid perimenopausal women with high-normal TSH levels. Corruble et al. (
29) observed poorer antidepressant response, especially to selective serotonin reuptake inhibitors, in patients with major depression and higher TSH levels, with no correlation of response to serum T
3 or T
4 levels. Not all studies have found associations between TSH level and response (
30), but given relatively small numbers of patients available for evaluation in any clinic, none of these studies has been large enough to be definitive.
In assessing thyroid state, there has been some debate as to whether serum TSH level always accurately reflects brain thyroid hormone levels, as transporters vary somewhat among different tissues and even in different cells within tissues, such as glia and neurons (
31,
32). However, there are numerous uptake proteins, providing compensatory mechanisms for transport (
32). Known abnormalities of brain uptake with clinically documented consequences are rare—for example, genetic disorders associated with peripheral and brain anomalies and with developmental disorders and substantial disability (
32,
33). Of note in interpreting TSH levels is that uptake of thyroid hormone is best in the pituitary, which has the most active transporters (
31). If thyroid hormone levels in the pituitary are low and TSH level consequently is high, levels are likely to be even more inadequate in brain. Thus, a high serum TSH level requires serious consideration for treatment.
TSH measures vary by time of day, from day to day, and from lab to lab, making it important to obtain repeated measurements to monitor supplementation (
12,
13). Thyroid hormone supplementation that is adequate to relieve most signs and symptoms of hypothyroidism may not be adequate for treatment of patients with depression. A well-powered study (with 13,000 subjects enrolled) assessing this issue (
34) observed a correlation between elevated TSH levels and depression, even with TSH levels within the usual laboratory range. Specifically, the depression rate was twice as high in women with TSH levels above 2.3 μIU/mL, again suggesting that the optimal TSH level is well below 4 μIU/mL.
The second case vignette presented above, a recent case, in which “on/off” treatment was prescribed, illustrates that for some patients, titrating thyroid hormone to a TSH level below 2.5 μIU/mL may be crucial in amelioration of symptoms.
Clinical Intervention: Choice, Dosage, and Monitoring of thyroid Hormone Supplementation
There is a large literature dating back to the 1980s on the use of thyroid hormone supplementation in depression, either given alone or as augmentation to an antidepressant medication, even when there is no clear evidence of thyroid dysfunction. The results of those studies are reviewed in detail in Lasser and Baldessarini (
7) and Jonklass et al. (
25). Most studies suggest only modest benefit from thyroid hormone supplements given as the primary treatment for depression or even as augmentation of standard antidepressant treatment in patients with depression in the absence of thyroid dysfunction. However, some studies suggest that supplementation with either T
4 (
39) or T
3 (
40,
41) may exert effects comparable to lithium as augmentation for antidepressants (
39,
40). Unfortunately, these studies have been too few and too small to be definitive (
42).
By comparison, studies of thyroid hormone supplementation in patients with documented thyroid abnormalities show consistently positive effects (
25). Questions have arisen about whether T
3 or T
4 might be preferable as thyroid supplementation in depressed patients. Most often, endocrinologists prescribe T
4 (usually its synthetic version, levothyroxine, because supplements prepared from natural sources show high variability) (
43), and studies have shown no advantages for T
3 (also its synthetic version, liothyronine) (
14). However, in the depression literature, there are more studies of T
3. T
3 may enter and equilibrate in tissue more rapidly than T
4, and in one small study comparing the effects of T
3 and T
4, the results favored T
3, although patients in each group appeared to respond to the hormone they received (
44). The brain preferentially takes up T
4, and Joffee (
45) suggested that T
3 supplementation may decrease serum T
4 by feedback inhibition in the hypothalamus, thus decreasing brain T
3. No confirmatory studies have been conducted to test this hypothesis. Notably, a study by Targum et al. (
46) comparing T
4 and T
3 showed similar beneficial effects with either of these supplements.
In a related matter, it has been argued that abnormalities of transport or conversion of T
4 to T
3 may produce hypothyroidism only in the brain and not in peripheral tissues (
47), but no evidence supports this speculation for depression. Uptake of T
4 and T
3 occurs by multiple mechanisms, and none of the transport or metabolic mechanisms is unique to the brain. Either T
4 or T
3 supplementation produces adequate active T
3 in the brain, and variants of transport or metabolism of thyroid hormone leading to disease are rare and are not likely to produce specific effects on mood (
48).
Thus, to date, few studies comparing T
3 and T
4 have been conducted, none of them adequately powered, and no compelling clinical or physiologic evidence is available to favor T
3 or T
4 as a treatment (
25,
42). T
3 is absorbed faster, but this can be a disadvantage, as this supplement may have to be taken more than once a day, and rapid absorption can lead to mild and transient hyperthyroidism after some doses. Moreover, thyroid hormone often must be taken a half hour before meals to avoid fluctuations in blood levels, making multiple doses per day unwieldy (
49). There are no clinical features or markers that might be used to choose a particular thyroid hormone supplement (
50). T
4, as the most commonly prescribed supplement in internal medicine, is a reasonable choice. On the other hand, because most depression studies of thyroid hormone supplementation have used T
3, it is also reasonable to start with that. If a patient is already receiving either T
4 or T
3, increasing the dosage of that supplement is the most straightforward choice. Although some clinicians have prescribed T
3 for patients already receiving T
4, there are reports but no definitive trials supporting that approach (
51).
There are other clinical considerations in the prescription of thyroid hormone. If a patient has switched brands or preparations of thyroid supplement, lowered or increased tissue levels and effects may ensue (
14). If a patient taking an increased dosage of T
4 experiences no amelioration of depressive symptoms, a trial or addition of T
3 is reasonable. Low dosages, starting with 50–100 μg/day for T
4 (levothyroxine) and 25–50 μg/day for T
3 (liothyronine) usually suffice (
52), but it is important to track symptoms, side effects, and serum TSH levels.
Elevated thyroid hormone levels have been associated with a variety of side effects, including increased pulse rate, decreased appetite and sleep, gastrointestinal hyperactivity, and agitation, but at the dosages of thyroid hormone used to augment antidepressants, these side effects are usually mild at worst. The most common worrisome side effect of thyroid hormone treatment is atrial fibrillation, but that is rare and is usually seen only if serum TSH is driven very low, below 0.1 μIU/mL (
14). Therefore, TSH level should be driven below 2 μIU/mL, unless side effects, such as agitation, intervene. In our own practices, unless response is seen at lower dosages, we often attempt to achieve enough thyroid hormone supplementation to drive the TSH to 1.0 μIU/mL or just below, in order to give supplementation an adequate trial. Beneficial effects often appear early, sometimes within a week, but an adequate trial requires 6 weeks, and full effects may require months (
14).
Summary and Conclusions
Adequate thyroid hormone levels are necessary for most physiologic functions, including normal brain function and response to medications. Unfortunately, the literature is sparse on the specific topic of treating patients who have both high-normal TSH levels, suggesting some degree of thyroid inadequacy, and major depressive disorder. Most studies addressing the use of thyroid hormone supplementation in patients with mild thyroid dysfunction and depression have been small, often called “pilot” and “preliminary” studies by their authors. Such studies have not been followed by large-scale definitive studies, and despite the clinical importance of defining adequate thyroid function in refractory depression, it is unlikely that definitive large-scale studies will be conducted.
Likewise, the cases we cited here are not proof of best treatment, but rather they illustrate the clinical importance of optimizing TSH level in a manner consistent with the evidence summarized from the literature. Ultimately, one must make choices when standard treatment is not adequately effective.
Some conclusions can be drawn from the literature and clinical experience. Overt thyroid disease or subclinical hypothyroidism is not the cause of most depression, and thyroid hormone supplementation alone is not an effective treatment for most cases of depression. However, mild thyroid abnormalities or inadequate thyroid hormone supplementation are not rare in patients with depression, and a target TSH level below 4.5 μIU/mL, as previously recommended in the literature, would appear to be inadequate for most people, especially those with treatment-resistant depression. In monitoring supplementation, both blood tests and side effects are followed, but it is likely that the TSH level will need to be below 2.5 μIU/mL, or even below 2 μIU/mL, for most patients to achieve optimal outcomes.
The literature strongly suggests that patients with high-normal TSH levels or subclinical hypothyroidism and clinical complaints, including depression, benefit from additional thyroid hormone. Fortunately, thyroid supplementation comes with few side effects and can be adjusted easily, so the recommendation of increased supplementation in instances of high-normal TSH levels and poor antidepressant response is a low-risk intervention.
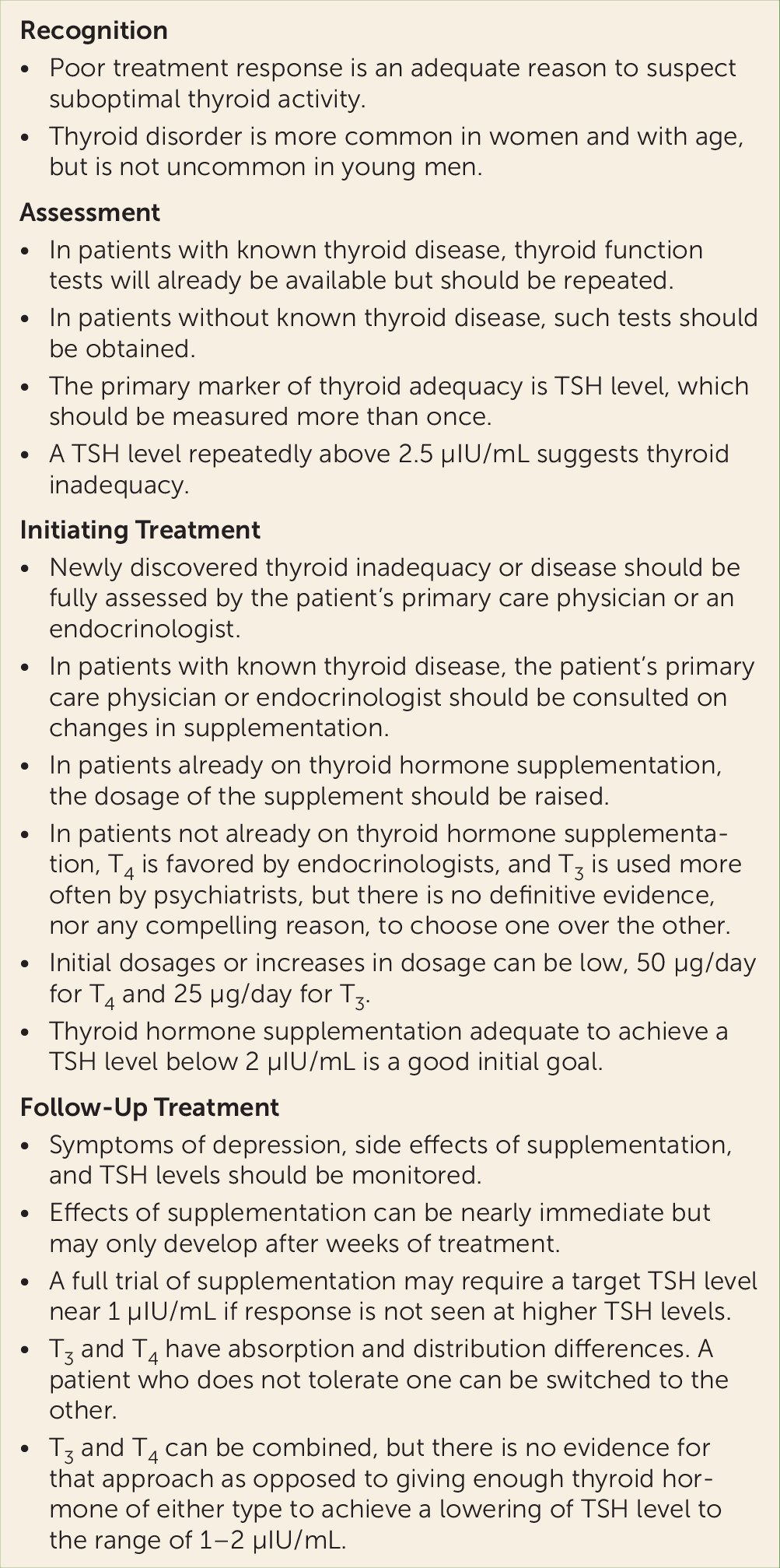
Guidelines for the evaluation and treatment of patients with known or suspected thyroid dysfunction and depression are summarized in
Figure 2. Rosenthal et al. (
58) have also provided useful considerations previously in these pages. Readers should note that the TSH levels we have quoted here are the most common laboratory ranges, but some laboratories may use different assays and have different normal ranges. In any case, results at the higher end of the laboratory normal range may indicate suboptimal thyroid hormone levels in the patient tested (
12).