Physical Activity and Incident Depression: A Meta-Analysis of Prospective Cohort Studies
Abstract
Objective:
Method:
Results:
Conclusions:
Method
Search Procedure
Inclusion and Exclusion Criteria
Study Selection
Outcomes
Data Extraction
Study Quality
Meta-Analysis
Results
Search Results
Studies and Participant Characteristics
| Study Authors, Year, Reference | Study Name | Country | N | Follow-Up (years) | Persons-Years | Male (%) | Age at Baseline | Depression Definition | Physical Activity Measure | Physical Activity Parameters |
|---|---|---|---|---|---|---|---|---|---|---|
| Almeida et al., 2006 (29)b,c,d | None | Australia | 601 | 4.8 | 2,884 | 100.0 | Older | GDS-15 >5 | Single question on physical activity intensity | Intensity |
| Augestad et al., 2008 (30)e | Nord-Trøndelag Health Survey (HUNT) | Norway | 6,660 | 11 | 73,271 | 49.6 | Adults | HADS-D >8 | Physical activity questions used in HUNT study | Composite/metabolic equivalents |
| Baumeister et al., 2017 (31)e,f,g | Study of Health in Pomerania (SHIP) | Germany | 1,952 | 4.5 | 8,784 | 49.7 | Adults | BDI-II ≥12 or M-CIDI | Baecke questionnaire | Frequency |
| Beard et al., 2007 (32)c | Northern Rivers Mental Health Study (NoRMHS) | Australia | 968 | 2 | 1,936 | 43.3 | Adults | CIDI or DIS | Physical activity questionnaire (not specified) | Volume |
| Brown et al., 1996 (33)b,c,h | National Health and Nutrition Examination Survey (NHANES I) | United States | 1,132 | 7–9 (8 used) | 9,056 | 45.0 | Adults | CES-D ≥16 | Two questions on level of activity | N/A |
| Cabello et al., 2017 (34)d,e | WHO Study on Global Ageing and Adult Health (SAGE) | Ghana, India, Mexico, and Russian Federation | 4,888 | 3 (computed) | 14,664 | 34.5 | Adults | CIDI-based algorithm | IPAQ | Composite/metabolic equivalents |
| Chang et al., 2016 (35)e | Age Gene/Environment Susceptibility–Reykjavik Study | Iceland | 4,140 | 25 | 103,500 | 55.4 | Older | GDS-15 >6 | Two questions on regularity and time spent in physical activity | Volume |
| Chang et al., 2016 (36)b,c,d,f,h | Nurses’ Health Study (NHS) Waves 2000–2010 | United States | 21,728 | 10 | 217,280 | 0.0 | Older | Self-report of physician-diagnosed major depression or depressive symptoms, use of antidepressants, MHI-5 <52, CES-D10 ≥16 or GDS-15 >6 (2000–2010) | Questions on hours per week of moderate to vigorous exercise | Volume |
| Chen and Millar, 1999 (37)b,c,d,h,i | National Population Health Survey (NPHS 1994/1995–1996/1997) | Canada | 7,593 | 2 | 15,186 | 46.2 | Adults | CIDI | Questions on frequency and duration of different physical activities | Composite/metabolic equivalents |
| Choi et al., 2015 (38)c,d,e | Korean Longitudinal Study of Aging (KLoSA) | South Korea | 5,327 | 4 | 21,308 | 47.4 | Adults | CES-D10 ≥4 | Single question on exercise | Frequency |
| Clark et al., 2007 (39)d,e | East London Adolescents: Community Health Survey (RELACHS) | England | 1,170 | 2 | 2,340 | 49.5 | Children/adolescents | SMFQ ≥8 | Question on physical activity/exercise | Unclear |
| Collard et al., 2015 (40)d,f | Invecchiare in Chianti (Aging in the Chianti Area) (InCHIANTI) | Italy | 699 | 9 | 6,291 | 50.0 | Older | CES-D ≥20 | Unclear | Unclear |
| Cooper-Patrick et al., 1997 (41)b,d,f | Precursors Study | United States | 752 | 15 | 11,280 | 92.0 | Adults | Self-report on annual morbidity questionnaires and by review of medical records, using DSM-IV criteria | Harvard Alumni Physical Activity Questionnaire | Frequency |
| Da Silva et al., 2012 (42)c,e | Whitehall II Study | United Kingdom | 9,309 | 8 | 74,472 | 68.5 | Adults | GHQ (four items for depression) ≥ 4 | Two questions on time and intensity of physical activity | Composite/metabolic equivalents |
| España-Romero et al., 2013 (43)e | Aerobic Center Longitudinal Study (ACLS) | United States | 5,110 | 6.1 | 31,171 | 79.6 | Adults | CES-D ≥8 | Participation in recreational physical activity | Composite/metabolic equivalents |
| Farmer et al., 1988 (44)d,e | National Health and Nutrition Examination Survey (NHANES I) augumentation group (1975) | United States | 1,163 | 7–9 (8 used) | 9,304 | 48.5 | Adults | CES-D ≥16 | Two questions on level of activity | N/A |
| Gallegos-Carrillo et al., 2013 (45)e | Health Worker Cohort Study (HWCS) | Mexico | 1,047 | 6 | 6,282 | 22.5 | Adults | CES-D ≥16 | Questionnaire assessing time and intensity spent in different recreational physical activities | Composite/metabolic equivalents |
| García-Peña et al., 2013 (46)e | Integrated Study of Depression Among the Elderly | Mexico | 7,449 | 2 | 14,898 | 48.85 | Older | GDS-30 >11 | Single question on regular exercise | Unclear |
| Giltay et al., 2006 (47)b,c,h | Zutphen Elderly Study | Netherlands | 464 | 15 | 6,960 | 100.0 | Older | ZSDS ≥50 | Questionnaire on total minutes per week | Volume |
| Groffen et al., 2013 (48)d,f | Health, Aging, and Body Composition (Health ABC) Study | United States | 2,694 | 9 | 24,246 | 49.7 | Older | CES-D10 >10 or use of antidepressant medication | Adapted Minnesota Leisure Time Physical Activity Questionnaire | Composite/metabolic equivalents |
| Hiles et al., 2015 (49)e,g | Hunter Community Study | Australia | 1,410 | 4 | 5,640 | 49.6 | Older | CES-D ≥16 | Pedometer | Volume |
| Jerstad et al., 2010 (50)e,g | None | United States | 496 | 6 | 2,976 | 0.0 | Children/adolescents | K-SADS | Past Year Activity Scale | Volume |
| Jonsdottir et al., 2010 (51)b,f | None | Sweden | 2,818 | 2 | 5,636 | 13.0 | Adults | HADS-D > 10 | Adapted Saltin-Grimby scale | Intensity |
| Joshi et al., 2016 (52)e,g | New York City Neighborhood and Mental Health in the Elderly Study II | United States | 2,355 | 3 | 7,065 | 40.1 | Older | PHQ-9 ≥10 | PASE | Composite/metabolic equivalents |
| Koster et al., 2006 (53)d,f | Longitudinal Aging Study Amsterdam (LASA) | Netherlands | 2,153 | 9 | 19,377 | 50.8 | Older | CES-D ≥16 | Question on number of activities in past week | Frequency |
| Ku et al., 2009 (54)e,g | Taiwan’s Health and Living Status of the Elderly Survey | Taiwan | 3,778 | 7 | 26,446 | 53.9 | Older | CES-D10 ≥10 | Single question on frequency of leisure-time physical activity | Frequency |
| Kuwahara et al., 2015 (55)b,f | None | Japan | 29,802 | 6.4 | 190,732 | 84.8 | Adults | Adapted CES-D ≥16 | Questions on regularity, frequency, and time spent in 20 physical activities | Composite/metabolic equivalents |
| Mckercher et al., 2014 (56)b,c,f,h | Childhood Determinants of Adult Health Study | Australia | 1,630 | 20 | 32,600 | 46.5 | Children/adolescents | CIDI-Auto | Questions on frequency and time spent on physical activity | Composite/metabolic equivalents |
| Messier et al., 2013 (57)b,c,d,e,h | Montreal Diabetes Health and Well-Being Study | Canada | 1,868 | 1 | 1,868 | 46.4 | Adults | PHQ-9 (one of the first two symptoms and five of the others) | Question on the frequency of sports participation | Frequency |
| Mihrshahi et al., 2015 (58)c,e | Australian Longitudinal Study on Women’s Health (ALSWH), waves 2004–2010 | Australia | 5,117 | 6 | 30,702 | 0.0 | Adults | CES-D10 ≥10 | Questionnaire based on Australian recommendations for physical activity | Composite/metabolic equivalents |
| Mikkelsen et al., 2010 (59)b,f,g | Copenhagen City Heart Study | Denmark | 18,146 | 26 | 471,796 | 44.1 | Adults | Record of major depression in Danish hospital discharge register or Danish psychiatric hospital (ICD-8 codes 296, 298, or 300 or ICD-10 codes F32, F33) | Single question on intensity and frequency | Composite/metabolic equivalents |
| Mobily et al., 1996 (60)e | Iowa 65+ Rural Health Study | United States | 1,926 | 10 | 19,260 | 38.6 | Older | CES-D11 ≥15 | Single question on walking frequency | Frequency |
| Park et al., 2015 (61)b,c,e,h | Yeoncheon Elderly Depression and Dementia Study | South Korea | 340 | 5 | 1,700 | 61.2 | Older | SGDS-K ≥8 | IPAQ | Composite/metabolic equivalents |
| Pasco et al., 2011 (62)e | Geelong Osteoporosis Study (GOS) | Australia | 547 | 4.1 | 2,242 | 56.0 | Older | SCID-I/NP | PASE | Composite/metabolic equivalents |
| Rius-Ottenheim et al., 2013 (63)b,c,e,h | Alpha Omega Trial (AOT) | Netherlands | 445 | 4.3 | 1,913 | 81.3 | Older | GDS-15 ≥4 | PASE | Composite/metabolic equivalents |
| Roh et al., 2015 (64)e | None | Korea | 15,146 | 3 | 45,438 | 44.5 | Older | SGDS-K ≥8 | Two questions on frequency and duration of moderate to vigorous physical activity “in a week” | Frequency |
| Sanchez-Villegas et al., 2008 (65)e | SUN study | Spain | 10,381 | 6 | 62,286 | N/A | Adults | Self-report of physician diagnosis of depression | Questionnaire assessing time spent per week in 17 physical activities | Composite/metabolic equivalents |
| Sanchez-Villegas et al., 2016 (66)d,f | SUN study | Spain | 11,800 | 14 | 165,200 | N/A | Adults | Self-report of physician diagnosis of depression | Questionnaire assessing time spent per week in 17 physical activities | Composite/metabolic equivalents |
| Smith et al., 2010 (67)c,e | Honolulu-Asia Aging Study | United States | 1,417 | 8 | 11,336 | 100.0 | Older | CES-D11 ≥9 | Single question on distance walked per day | Volume |
| Strawbridge et al., 2002 (68)d,e | Alameda County Study (waves 1994–1999) | United States | 1,651 | 5 | 8,255 | N/A | Older | DSM-12D | Four questions evaluating the usual frequency of physical exercise, taking part in active sports, taking long walks, and swimming | Frequency |
| Strohle et al., 2007 (69)e | Early Developmental Stages of Psychopathology Study (EDSP) | Germany | 2,458 | 4 | 9,832 | 50.9 | Children/adolescents | CIDI | Four questions on physical activity frequency | Frequency |
| Ten Have et al., 2011 (70)e | Netherlands Mental Health Survey and Incidence Study (NEMESIS) | Netherlands | 4,796 | 3 | 14,388 | 50.6 | Adults | DSM-III-R | Single question on hours per week of exercise | Volume |
| Tsai et al., 2013 (71)e | Taiwan Longitudinal Survey on Aging (TLSA) | Taiwan | 2,145 | 8 | 17,160 | 53.2 | Older | CES-D ≥10 | Three questions on frequency, time, and intensity of exercise | Composite/metabolic equivalents |
| Tsutsumimoto et al., 2017 (72)b,c,d,e,h | Obu Study of Health Promotion for the Elderly (OSHPE) | Japan | 3,053 | 15 | 45,795 | 49.7 | Older | GDS-15 >6 | IPAQ | Volume |
| Veronese et al., 2017 (73)c,d,e | English Longitudinal Study of Ageing (ELSA) | United Kingdom | 4,077 | 2 | 8,154 | 47.0 | Older | CES-D8 ≥4 | Three questions on frequency of participation in light, moderate, or vigorous physical activity | Intensity |
| Wang et al., 2011 (74)d,f | National Population Health Survey (NPHS waves 1994–1995 to 2004–2005) | Canada | 15,201 | 6 | 91,206 | 54.4 | Adults | CIDI | Questions on frequency and duration of engagement in different physical activities | Composite/metabolic equivalents |
| Weyerer, 1992 (75)d,e | Upper Bavarian Field Study | Germany | 1,233 | 5 | 6,165 | 46.8 | Adults | CIS | Single question on exercise frequency | Frequency |
| Wise et al., 2006 (76)b,c,e | Black Women’s Health Study | United States | 35,224 | 2 | 70,448 | 0.0 | Adults | CES-D ≥16 | Questionnaire on number of hours spent in walking for exercise and vigorous exercise | Volume |
| Yoshida et al., 2015 (77)c,d,e | None | Japan | 680 | 1 | 680 | 42.5 | Older | GDS-15 >6 | Questions about participants’ engagement in physical activity and weekly frequency | Frequency |
Study Quality
Physical Activity and Incident Depression
Highest versus lowest physical activity.
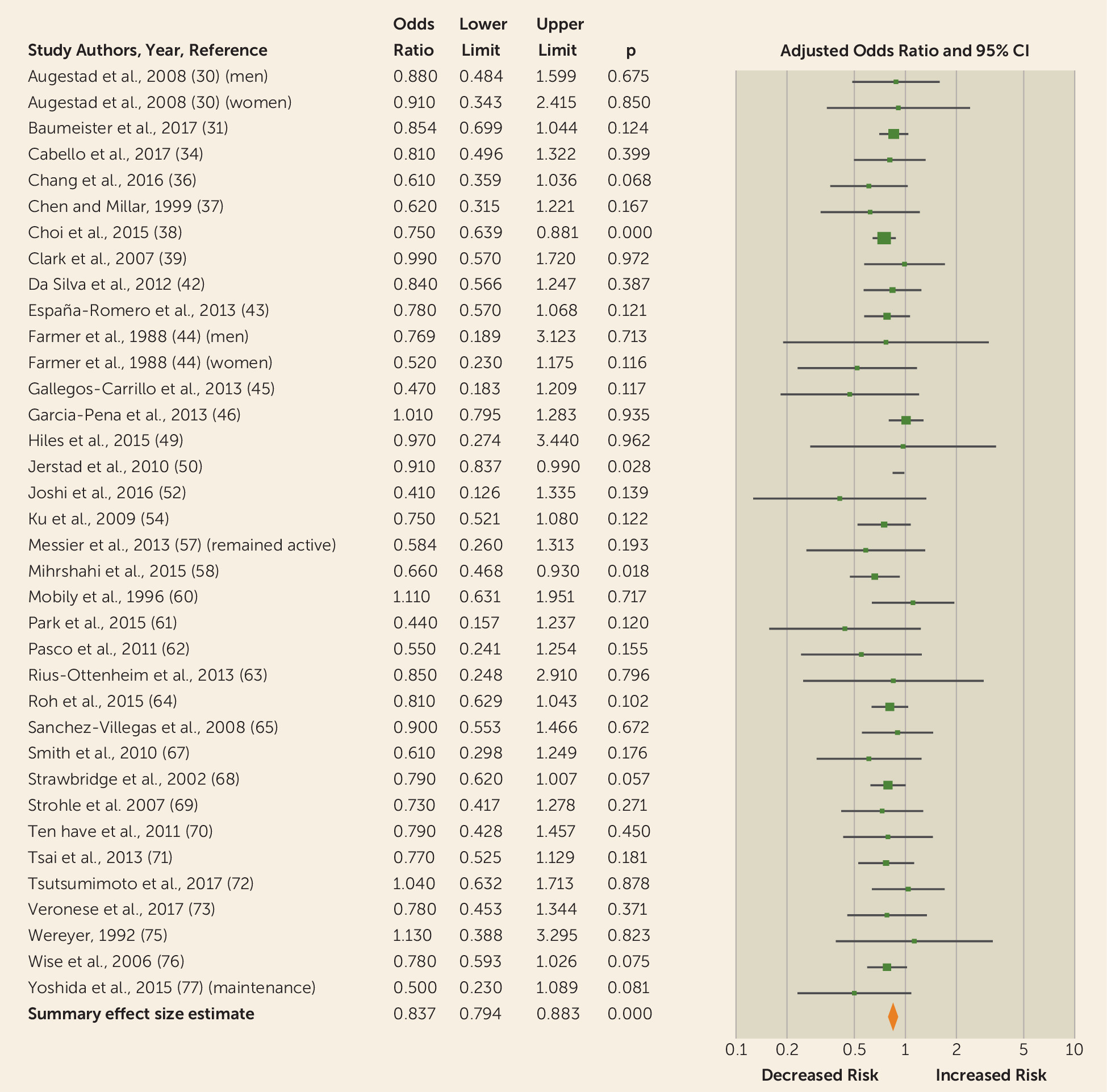
Subgroup and sensitivity analysis.
| Analysis | Number of Cohorts (Arms) | Meta-Analysis | Heterogeneity | Trim and Fill Method | Adjusted Studies | Classic Fail Safe N | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Studies with adjusted odds ratio | Adjusted odds ratio | 95% CI | p | I2 | Q | Effect size | 95% CI | N | N | |
| Overall | 36 | 0.837 | 0.794, 0.883 | <0.0001 | 0.00 | 25.93 | 0.85 | 0.81, 0.89 | 10 | 380 |
| Continent | ||||||||||
| Asia | 7 | 0.765 | 0.682, 0.859 | <0.0001 | 0.00 | 3.97 | 0.77 | 0.69, 0.87 | 2 | 26 |
| Europe | 12 | 0.836 | 0.732, 0.954 | 0.008 | 0.00 | 2.53 | 0.72 | 0.72, 0.94 | 1 | 6 |
| North America | 13 | 0.864 | 0.796, 0.937 | <0.0001 | 4.28 | 12.53 | 0.88 | 0.79, 0.97 | 6 | 54 |
| Oceania | 3 | 0.658 | 0.484, 0.895 | 0.008 | 0.00 | 0.54 | 0.64 | 0.47, 0.86 | 1 | 1 |
| Physical activity assessment unit | ||||||||||
| Composite/metabolic equivalents | 14 | 0.746 | 0.648, 0.858 | <0.0001 | 0.00 | 5.83 | 0.75 | 0.66, 0.87 | 2 | 50 |
| Frequency | 10 | 0.789 | 0.718, 0.866 | <0.0001 | 0.00 | 4.85 | Unchanged | 39 | ||
| Intensity | 1 | 0.780 | 0.453, 1.344 | 0.371 | 0.00 | 0.00 | N/A | N/A | ||
| Volume | 7 | 0.888 | 0.822, 0.960 | 0.003 | 0.00 | 4.71 | 0.89 | 0.83, 0.97 | 2 | 9 |
| 150 minutes of moderate to vigorous physical activity per week | 4 | 0.780 | 0.617, 0.986 | 0.038 | 0.00 | 1.33 | 0.77 | 0.61, 0.97 | 2 | 1 |
| Depression assessment | ||||||||||
| Depressive symptoms | 28 | 0.844 | 0.798, 0.892 | <0.0001 | 0.00 | 23.22 | 0.85 | 0.81, 0.90 | 7 | 245 |
| Major depression | 10 | 0.862 | 0.757, 0.981 | 0.024 | 0.00 | 5.29 | 0.89 | 0.79, 1.00 | 3 | 7 |
| Age at baseline | ||||||||||
| Adults | 16 | 0.787 | 0.707, 0.877 | <0.0001 | 0.00 | 5.85 | 0.79 | 0.71, 0.88 | 1 | 57 |
| Older | 16 | 0.794 | 0.726, 0.868 | <0.0001 | 0.00 | 13.13 | 0.80 | 0.74, 0.88 | 4 | 85 |
| Children/adolescents | 3 | 0.907 | 0.836, 0.985 | 0.021 | 0.00 | 0.68 | Unchanged | 0 | ||
| Adjustments | ||||||||||
| Age and sex | 32 | 0.836 | 0.791, 0.883 | <0.0001 | 0.00 | 20.92 | 0.85 | 0.80, 0.90 | 10 | 310 |
| a. Baseline depressive symptoms | 3 | 0.897 | 0.829, 0.970 | 0.007 | 0.00 | 0.99 | Unchanged | 3 | ||
| b. Body mass index | 12 | 0.871 | 0.810, 0.937 | <0.0001 | 0.00 | 8.18 | 0.90 | 0.81, 1.00 | 5 | 34 |
| c. smoking | 12 | 0.748 | 0.647, 0.865 | <0.0001 | 0.00 | 6.37 | 0.75 | 0.65, 0.87 | 1 | 32 |
| Age and sex and one other (a, b, or c) | 17 | 0.865 | 0.800, 0.928 | <0.0001 | 0.00 | 12.38 | 0.88 | 0.80, 0.97 | 6 | 66 |
| Age and sex and two others (a+b, a+c, or b+c) | 8 | 0.836 | 0.749, 0.934 | 0.001 | 9.23 | 7.71 | 0.90 | 0.79, 1.03 | 5 | 26 |
| Studies with crude odds ratio | Odds ratio | 95% CI | p | I2 | Q | Effect size | 95% CI | N | N | |
| Overall | 19 | 0.591 | 0.510, 0.685 | <0.0001 | 52.38 | 37.80 | 0.63 | 0.54, 0.74 | 4 | 519 |
| Continent | ||||||||||
| Asia | 4 | 0.657 | 0.577, 0.749 | <0.0001 | 0.00 | 2.01 | 0.66 | 0.58, 0.75 | 1 | 24 |
| Europe | 4 | 0.546 | 0.286, 1.040 | 0.065 | 75.01 | 12.00 | 0.37 | 0.19, 0.73 | 2 | 13 |
| North America | 6 | 0.644 | 0.496, 0.835 | 0.001 | 63.94 | 13.86 | Unchanged | 52 | ||
| Oceania | 5 | 0.480 | 0.405, 0.568 | <0.0001 | 0.00 | 0.07 | 0.48 | 0.40, 0.56 | 1 | 35 |
| Studies with crude odds ratio | Odds ratio | 95% CI | p | I2 | Q | Effect size | 95% CI | N | N | |
| Physical activity assessment unit | ||||||||||
| Composite/metabolic equivalents | 4 | 0.574 | 0.402, 0.819 | 0.002 | 52.52 | 6.31 | Unchanged | 33 | ||
| Frequency | 3 | 0.662 | 0.580, 0.755 | <0.0001 | 0.00 | 0.36 | 0.66 | 0.58, 0.75 | 1 | 17 |
| Intensity | 2 | 0.303 | 0.198, 0.462 | <0.0001 | 0.00 | 0.53 | N/A | N/A | ||
| Volume | 8 | 0.628 | 0.487, 0.810 | <0.0001 | 48.49 | 13.59 | 0.64 | 0.51, 0.80 | 2 | 56 |
| 150 minutes of moderate to vigorous physical activity per week | 3 | 0.704 | 0.477, 1.038 | 0.077 | 8.38 | 2.18 | Unchanged | 1 | ||
| Depression assessment | ||||||||||
| Depressive symptoms | 14 | 0.618 | 0.568, 0.674 | <0.0001 | 58.44 | 31.28 | 0.66 | 0.54, 0.80 | 3 | 281 |
| Major depression | 5 | 0.511 | 0.429, 0.608 | <0.0001 | 0.00 | 2.79 | 0.48 | 0.40, 0.58 | 2 | 32 |
| Age at baseline | ||||||||||
| Adults | 8 | 0.662 | 0.550, 0.979 | <0.0001 | 62.10 | 18.47 | 0.63 | 0.54, 0.74 | 1 | 190 |
| Older | 9 | 0.496 | 0.399, 0.616 | <0.0001 | 21.79 | 10.22 | 0.45 | 0.36, 0.57 | 2 | 112 |
| Children/adolescents | 2 | 0.496 | 0.208, 1.186 | <0.0001 | 0.00 | 0.05 | N/A | N/A | ||
| Studies with adjusted relative risk/hazard ratio (ARR/AHR) | ARR/AHR | 95% CI | p | I2 | Q | Effect size | 95% CI | N | N | |
| Overall | 18 | 0.832 | 0.762, 0.909 | <0.0001 | 0.00 | 14.86 | 0.86 | 0.78, 0.96 | 8 | 102 |
| Continent | ||||||||||
| Asia | 1 | 0.950 | 0.777, 1.162 | 0.611 | 0.00 | 0.00 | N/A | N/A | ||
| Europe | 8 | 0.773 | 0.660, 0.906 | 0.001 | 21.92 | 8.99 | 0.84 | 0.70, 1.01 | 4 | 31 |
| North America | 7 | 0.811 | 0.673, 0.978 | 0.028 | 0.00 | 2.19 | 0.86 | 0.73, 1.02 | 3 | 4 |
| Oceania | 2 | 0.502 | 0.241, 1.045 | 0.0001 | 0.00 | 0.01 | N/A | N/A | ||
| Physical activity assessment unit | ||||||||||
| Composite/metabolic equivalents | 12 | 0.832 | 0.741, 0.935 | 0.002 | 0.00 | 8.72 | 0.88 | 0.79, 0.98 | 6 | 35 |
| Frequency | 3 | 0.873 | 0.755, 1.010 | 0.062 | 0.00 | 0.61 | 0.89 | 0.77, 1.02 | 2 | 0 |
| Intensity | 1 | 0.290 | 0.104, 0.805 | 0.017 | 0.00 | 0.00 | N/A | N/A | ||
| Volume | 1 | 0.815 | 0.815, 1.331 | 0.413 | 0.00 | 0.00 | N/A | N/A | ||
| 150 minutes of moderate to vigorous physical activity per week | 4 | 0.689 | 0.498, 0.951 | 0.024 | 0.00 | 0.53 | Unchanged | 2 | ||
| Depression assessment | ||||||||||
| Depressive symptoms | 11 | 0.845 | 0.766, 0.932 | 0.001 | 0.12 | 10.03 | 0.88 | 0.78, 1.00 | 6 | 57 |
| Major depression | 8 | 0.873 | 0.748, 1.108 | 0.082 | 10.78 | 7.84 | 0.93 | 0.77, 1.13 | 4 | 6 |
| Studies with adjusted relative risk/hazard ratio (ARR/AHR) | ARR/AHR | 95% CI | p | I2 | Q | Effect size | 95% CI | N | N | |
| Age at baseline | ||||||||||
| Adults | 9 | 0.863 | 0.776, 0.960 | 0.007 | 7.53 | 8.65 | 0.89 | 0.78, 1.02 | 4 | 20 |
| Older | 7 | 0.703 | 0.567, 0.879 | 0.001 | 0.00 | 1.27 | Unchanged | 12 | ||
| Children/adolescents | 0 | N/A | N/A | N/A | N/A | N/A | N/A | 0 | ||
| Adjustments | ||||||||||
| Age and sex | 18 | 0.832 | 0.762, 0.909 | <0.0001 | 0.00 | 14.86 | 0.86 | 0.78, 0.96 | 8 | 102 |
| a. Baseline depressive symptoms | 1 | 0.950 | 0.777, 1.162 | 0.618 | 0.00 | 0.00 | N/A | N/A | ||
| b. Body mass index | 10 | 0.821 | 0.714, 0.945 | 0.006 | 14.28 | 10.50 | 0.88 | 0.74, 1.04 | 5 | 28 |
| c. smoking | 4 | 0.694 | 0.505, 0.953 | 0.024 | 0.00 | 1.24 | Unchanged | 1 | ||
| Age and sex and (a, b, or c) | 11 | 0.833 | 0.734, 0.946 | 0.005 | 0.39 | 10.50 | 0.88 | 0.75, 1.04 | 5 | 29 |
| Age and sex and two others (a+b, a+c, or b+c) | 4 | 0.823 | 0.648, 1.045 | 0.109 | 13.25 | 11.23 | Unchanged | 1 | ||
| Studies with crude relative risk/hazard ratio (RR/HR) | RR/HR | 95% CI | p | I2 | Q | Effect size | 95% CI | N | N | |
| Overall | 17 | 0.687 | 0.601, 0.786 | <0.0001 | 33.40 | 24.02 | 0.80 | 0.69, 0.94 | 9 | 210 |
| Continent | ||||||||||
| Asia | 3 | 0.821 | 0.688, 0.980 | 0.029 | 0.00 | 1.00 | 0.84 | 0.72, 0.99 | 2 | 2 |
| Europe | 5 | 0.593 | 0.439, 0.801 | 0.001 | 0.00 | 3.10 | 0.55 | 0.42, 0.72 | 1 | 11 |
| North America | 6 | 0.681 | 0.537, 0.865 | 0.002 | 66.23 | 14.80 | Unchanged | 49 | ||
| Oceania | 3 | 0.513 | 0.270, 0.974 | 0.041 | 0.00 | 0.07 | 0.49 | 0.27, 0.91 | 1 | 0 |
| Physical activity assessment unit | ||||||||||
| Composite/metabolic equivalents | 5 | 0.774 | 0.653, 0.916 | 0.003 | 0.00 | 3.11 | 0.84 | 0.69, 1.01 | 3 | 9 |
| Frequency | 2 | 0.705 | 0.440, 1.129 | 0.146 | 0.00 | 0.00 | N/A | N/A | ||
| Intensity | 2 | 0.336 | 0.157, 0.718 | 0.005 | 0.00 | 0.48 | N/A | N/A | ||
| Volume | 6 | 0.695 | 0.538, 0.898 | 0.005 | 66.50 | 14.92 | 0.71 | 0.55, 0.90 | 1 | 40 |
| 150 minutes of moderate to vigorous physical activity per week | 2 | 0.635 | 0.368, 1.096 | 0.103 | 0.00 | 0.10 | N/A | N/A | ||
| Depression assessment | ||||||||||
| Depressive symptoms | 9 | 0.811 | 0.729, 0.920 | <0.0001 | 0.00 | 7.38 | 0.83 | 0.72, 0.95 | 5 | 36 |
| Major depression | 8 | 0.575 | 0.502, 0.660 | <0.0001 | 0.00 | 1.63 | 0.55 | 0.48, 0.63 | 3 | 63 |
| Age at baseline | ||||||||||
| Adults | 9 | 0.764 | 0.667, 0.876 | <0.0001 | 16.01 | 9.52 | 0.82 | 0.69, 0.96 | 5 | 51 |
| Older | 6 | 0.588 | 0.509, 0.678 | <0.0001 | 0.00 | 2.99 | 0.56 | 0.48, 0.64 | 2 | 34 |
| Children/adolescents | 2 | 0.537 | 0.250, 1.149 | 0.109 | 0.00 | 0.03 | N/A | N/A | ||
Meta-Regressions
| Moderator | Number of Cohorts | β | 95% CI | p | R2 |
|---|---|---|---|---|---|
| Studies presenting adjusted odds ratio | |||||
| Sample size | 36 | <–0.0001 | <–0.001, <0.001 | 0.44 | 0.00 |
| Year of publication | 36 | –0.0035 | <–0.015, <0.008 | 0.55 | 0.00 |
| Length of follow-up | 36 | 0.0001 | –0.018, 0.018 | 0.99 | 0.00 |
| Person-years | 36 | <–0.0001 | <–0.001, <0.001 | 0.13 | 0.00 |
| Number of covariates | 34 | –0.0183 | –0.035, <0.001 | 0.05 | 0.00 |
| Percent dropout | 29 | –0.0027 | –0.007, 0.001 | 0.23 | 0.00 |
| Study quality | 36 | 0.0105 | –0.067, 0.088 | 0.78 | 0.00 |
| Study quality (selection of participants) | 36 | 0.0657 | –0.161, 0.293 | 0.57 | 0.00 |
| Study quality (comparability) | 36 | 0.0080 | –0.185, 0.201 | 0.93 | 0.00 |
| Study quality (outcome) | 36 | 0.0777 | –0.039, 0.194 | 0.19 | 0.00 |
| Studies presenting crude odds ratio | |||||
| Sample size | 19 | <–0.0001 | <–0.001, <0.001 | 0.31 | 0.00 |
| Year of publication | 19 | –0.0251 | –0.049, <0.001 | 0.05 | 0.00 |
| Length of follow-up | 19 | –0.0025 | –0.035, 0.030 | 0.87 | 0.02 |
| Person-years | 19 | <–0.0001 | <–0.001, <0.001 | 0.48 | 0.00 |
| Percent males | 19 | 0.0002 | –0.003, 0.003 | 0.88 | 0.00 |
| Percent dropout | 16 | –0.0034 | –0.014, 0.007 | 0.52 | 0.00 |
| Study quality | 19 | –0.1168 | –0.409, 0.175 | 0.43 | 0.02 |
| Study quality (selection of participants) | 19 | –0.0705 | –0.450, 0.309 | 0.71 | 0.00 |
| Study quality (outcome) | 19 | –0.1364 | –0.601, 0.328 | 0.56 | 0.00 |
| Studies presenting adjusted relative risk/adjusted hazard ratio | |||||
| Sample size | 18 | <0.0001 | <–0.001, <0.001 | 0.13 | 0.00 |
| Year of publication | 18 | 0.0207 | –0.008, 0.049 | 0.16 | 0.00 |
| Length of follow-up | 18 | –0.0132 | –0.028, 0.001 | 0.08 | 0.00 |
| Person-years | 18 | <0.0001 | <–0.001, <0.001 | 0.59 | 0.00 |
| Number of covariates | 18 | 0.0195 | –0.009, 0.048 | 0.19 | 0.00 |
| Percent dropout | 15 | –0.0036 | –0.020, 0.013 | 0.67 | 0.00 |
| Study quality | 18 | –0.0139 | –0.132, 0.104 | 0.81 | 0.00 |
| Study quality (selection of participants) | 18 | 0.0010 | –0.268, 0.270 | 0.99 | 0.00 |
| Study quality (comparability)a | — | — | — | — | — |
| Study quality (outcome) | 18 | –0.0214 | –0.141, 0.098 | 0.72 | 0.00 |
| Studies presenting crude relative risk/hazard ratio | |||||
| Sample size | 17 | <0.0001 | <–0.001, <0.001 | 0.06 | 0.82 |
| Year of publication | 17 | –0.0036 | –0.026, 0.018 | 0.75 | 0.04 |
| Length of follow-up | 17 | –0.0124 | –0.029, 0.004 | 0.14 | 0.58 |
| Person-years | 17 | <–0.0001 | <–0.001, <0.001 | 0.70 | 0.07 |
| Percent males | 17 | 0.0010 | –0.002, 0.004 | 0.57 | 0.00 |
| Percent dropout | 13 | –0.0083 | –0.019, 0.003 | 0.15 | 0.00 |
| Study quality | 17 | –0.0580 | –0.134, 0.018 | 0.13 | 0.30 |
| Study quality (selection of participants) | 17 | 0.1204 | –0.059, 0.300 | 0.19 | 0.43 |
| Study quality (outcome) | 17 | –0.1033 | –0.362, 0.155 | 0.43 | 0.00 |
Discussion
Limitations and Future Research
Conclusions
Acknowledgments
Footnote
Supplementary Material
- View/Download
- 1.31 MB
- View/Download
- 42.50 KB
References
Information & Authors
Information
Published In
History
Keywords
Authors
Competing Interests
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

