Multiple longitudinal psychoradiological studies of treated patients document decreases in whole brain, frontal, and temporal lobe volumes, as well as increases in ventricle size, from 1 to 7 years following illness onset (
5,
9,
13,
14). Another line of work has reported both gray and white matter changes after antipsychotic treatment (
6,
7,
11,
15–
18). However, fewer studies have reviewed white matter changes in longitudinal studies (
14) and after antipsychotic treatment (
8,
19–
24), and most involve shorter-term follow-up. Psychoradiological studies of white matter change during short-term antipsychotic treatment have reported both increased (
23) and decreased fractional anisotropy (
8,
25) after 6 to 12 weeks of antipsychotic treatment. Over a 5-year follow-up of early-course patients, increased white matter alterations have been reported (
26). Cross-sectional studies suggest accelerated loss of white matter integrity with age in schizophrenia, but results are inconsistent, and again the potential influences of antipsychotic treatment remain uncertain (
4,
27–
37). Generally, while some clinical and preclinical data suggest that antipsychotic medications may have beneficial effects on white matter with chronic treatment (
36,
37), other data, especially after acute treatment in early-course patients, suggest adverse effects (
19,
20,
22).
A direct comparison of never-treated and treated long-term schizophrenia patients with a wide range of illness duration provides a tractable strategy for contrasting brain alterations in relation to illness duration and to test for differences related to treatment status. Although a cross-sectional study has limitations, such a study can provide information relative to important questions about course of illness and long-term drug effects on brain alterations in schizophrenia that is not otherwise available.
In developing countries such as China and India, there have been reports of neuroimaging findings in rare cases of never-treated long-term schizophrenia identified by community outreach programs in less affluent and rural areas (
38–
40). In a study of patients who were never treated for more than 5 years after illness onset, we demonstrated greater age-related cortical thinning in prefrontal and temporal gyri than in healthy comparison subjects (
40). In a study of 17 long-term never-treated schizophrenia patients with a mean illness duration of 15 years (SD=6) (
39), a limited reduction of fractional anisotropy in the left temporal lobe was observed relative to healthy comparison subjects. In this latter study with a relatively limited age range, no significant association was observed between white matter deficits and age or illness duration, and there was no comparison with treated patients.
In the present study, we conducted diffusion tensor imaging (DTI) studies with long-term never-treated schizophrenia patients, antipsychotic-treated patients matched on illness duration, and healthy comparison subjects. We hypothesized that more severe white matter deficits would be found in never-treated long-term schizophrenia patients relative to treated patients and that age-related decline in white matter tract integrity would be accelerated in never-treated patients relative to treated patients and healthy comparison subjects, especially in the corpus callosum.
Discussion
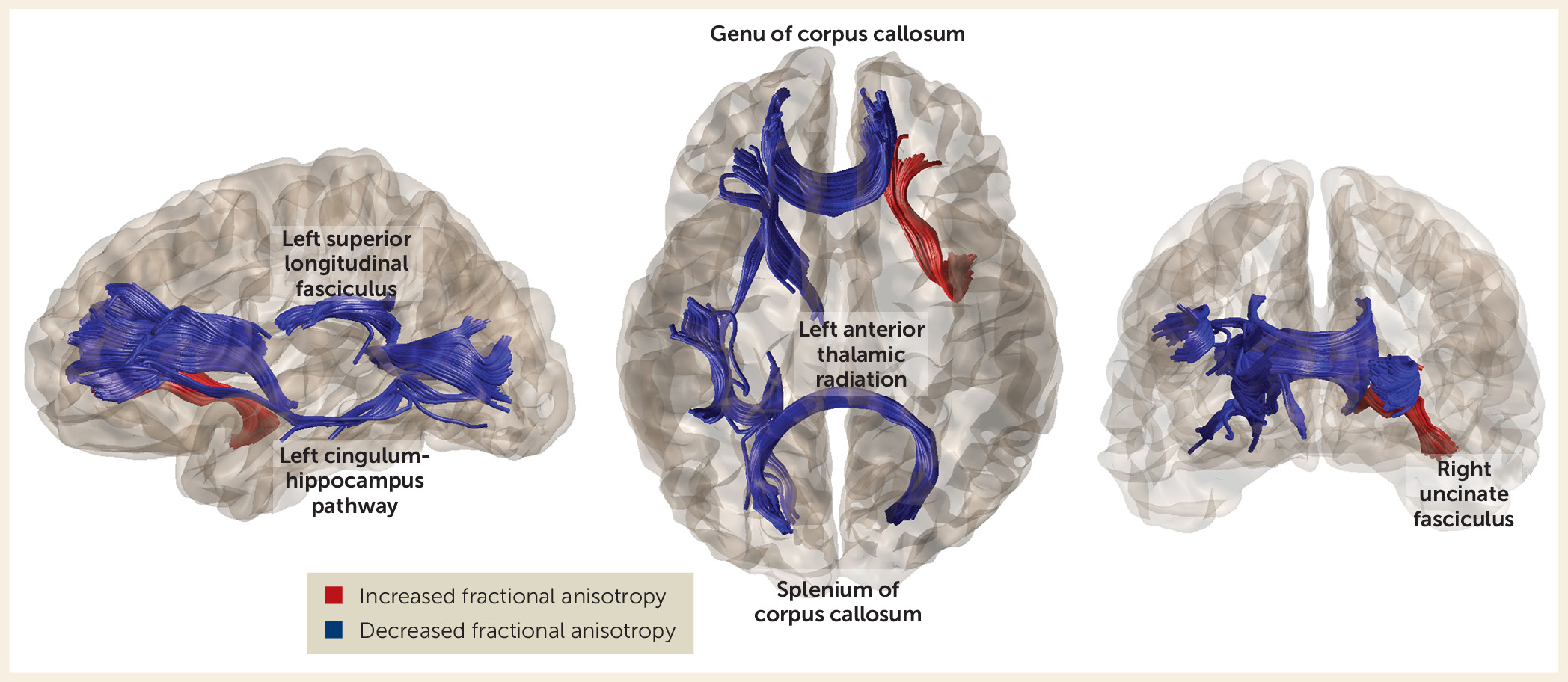
By comparing a rare group of never-treated long-term schizophrenia patients with both treated illness duration–matched patients and healthy comparison subjects, the present study demonstrated altered fractional anisotropy in both patient groups but greater fractional anisotropy alterations in never-treated patients. These alterations were predominantly in pathways of the frontal cortex (
Figure 1,
Table 2). In addition to extending previous reports of fractional anisotropy alteration in both treated and never-treated schizophrenia (
29,
39,
43,
44), we demonstrated a greater age-related reduction of fractional anisotropy in the genu of the corpus callosum in never-treated long-term schizophrenia patients relative to both healthy comparison subjects and antipsychotic-treated patients. Fractional anisotropy of the genu of the corpus callosum was related to symptom severity among never-treated patients. These findings provide novel insights into white matter abnormalities over the long-term course of illness without the influences of antipsychotic medication, and the findings regarding the anterior corpus callosum carry clinical relevance.
The greater reductions of fractional anisotropy in several fiber bundles throughout the brain in never-treated patients relative to treated patients suggest that long-term antipsychotic treatment may not only not cause adverse effects but may confer some beneficial effects on brain white matter via cumulative pharmacological effects or indirect benefits from treated psychosis, as has been argued previously (
43,
45,
46). Animal studies have shown that antipsychotics can facilitate oligodendrocyte regeneration and myelin repair following injury (
47). Because the oligodendrocyte abnormalities widely observed in postmortem studies of schizophrenia are thought to be one reason for decreased fractional anisotropy in white matter (
48,
49), this is one possible mechanism by which antipsychotic treatment might have beneficial effects on brain white matter. There are multiple direct and indirect mechanisms by which antipsychotic treatment may confer beneficial effects on white matter. Although the specific mechanisms remain to be identified, our findings of greater reduction of fractional anisotropy in never-treated patients relative to treated patients are consistent with a possible beneficial role of long-term antipsychotic treatment on brain white matter over the course of schizophrenia.
It is noteworthy that findings from the few previous short-term studies of antipsychotic medications on white matter integrity in schizophrenia are varied, with reports of increased fractional anisotropy (
23), decreased fractional anisotropy (
8,
25), and no changes (
24) after 6–12 weeks of acute antipsychotic treatment. Because our findings indicate greater fractional anisotropy reductions in never-treated patients than in treated patients across multiple tracts, it is possible that there are differences between early and longer-term antipsychotic treatment effects on white matter integrity. Acute treatment effects may be adverse, as suggested by some first-episode data (
8,
25) and by the somewhat lower fractional anisotropy values in younger treated patients in the present study. Longer-term treatment effects may be positive via cumulative pharmacologic effects, which may reduce adverse effects of persistent acute psychosis and improve multiple factors in general medical health over the illness course.
There are several significant challenges to studying very long-term effects of antipsychotic drugs on brain systems in patients. Besides the decades of follow-up required for longitudinal studies to examine such effects directly, there are problems of participant attrition, changes in MRI systems over time, difficulty controlling drug treatment plans, variable treatment adherence over a lifespan, and the fact that illness course and drug treatment are confounded because nearly all schizophrenia patients are treated once diagnosed, and dosage is related to illness severity. In the face of these challenges, the field lacks direct evidence about long-term effects of antipsychotic drugs on the brain in schizophrenia patients.
In this context, the present cross-sectional study of never-treated patients with long-term illness failed to detect evidence for an adverse effect of longer-term antipsychotic treatment. In addition, our data provide suggestive evidence supporting preclinical data indicating a potential beneficial effect of antipsychotic treatment—specifically, that it may help preserve white matter microstructure over the course of illness (
50,
51). Such an effect may in part explain the better overall clinical outcomes in antipsychotic-treated patients than in never-treated patients over the course of longer-term follow-up (
38).
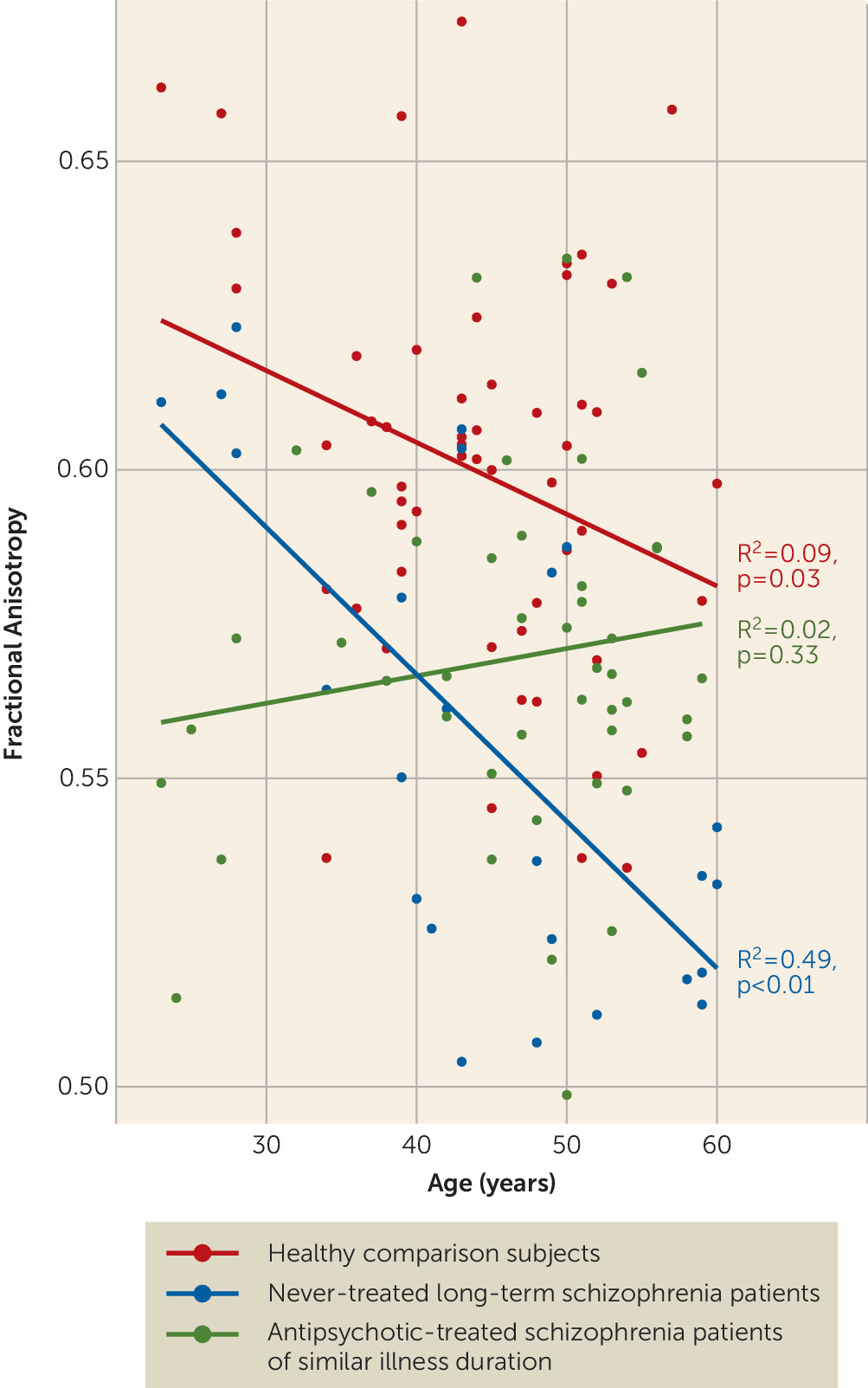
The greater age-related reduction of fractional anisotropy in the genu of the corpus callosum in never-treated patients than in treated patients and healthy comparison subjects suggests that in this circuitry, antipsychotic treatment effects might be particularly beneficial. The association of genu alterations with clinical symptoms in the present study and in previous reports (
52) strengthens this argument. Abnormalities in the genu have been reported previously (
27,
29,
43) and have been considered an important neurobiological observation because the tract provides most interhemispheric connectivity of the frontal lobes. This connectivity has long been known to influence cognitive and affective processes that are disrupted in schizophrenia (
43,
53). Previous observations of greater callosal alterations in chronic patients relative to first-episode patients (
54) are also consistent with the decline over the illness course seen in our never-treated patients.
Patterns observed in the genu of treated patients were notably distinct, as shown in
Figure 2. This group demonstrated little change with age, with fractional anisotropy appearing to be more reduced in the earlier years after illness onset compared with never-treated patients. This observation parallels that of a recent study in which healthy controls showed a modest fractional anisotropy decline with age while treated patients showed little age-related change. In that study, younger treated patients had lower fractional anisotropy than controls, but the difference disappeared with age (
27). One possible interpretation of these findings in relation to our own data is that any beneficial effects of antipsychotic treatment may be cumulative over the years of illness course or more pronounced later in the course of illness.
In our previous study with a partially overlapping sample of never-treated long-term patients, we observed an increased rate of age-related decline in cortical thickness in the ventral frontal cortex and superior temporal gyrus (
40). In the present study, while tracts in the temporal lobe had abnormal fractional anisotropy, the only differential age-related fiber tract effect in the never-treated patients was in the genu of the corpus callosum. This tract connects ventral prefrontal cortex regions across the hemispheres and thus may be related to previously reported accelerated age-related changes in ventral prefrontal cortical thickness.
Although findings of fractional anisotropy reduction were consistent across most tracts, an exception was the observation that the fractional anisotropy of the right uncinate fasciculus was higher in untreated patients than in treated patients. The uncinate fasciculus is a late-maturing pathway connecting the hippocampus and amygdala with the orbitofrontal cortex and thus is important for emotion and cognitive processing (
55). This finding may suggest a specific and different effect of antipsychotic treatment on this pathway, leading to decreased diffusion signaling along this fiber tract. While short-term effects on social cognition do not appear to be adverse (
56,
57), longer-term effects remain to be determined.
Several limitations should be considered when interpreting our findings. First, inferences that differences between the two patient groups are related to their treatment history are constrained by the lack of an experimental control (i.e., random assignment of patients to the two patient groups). Our approach may be perhaps the only feasible immediate-term strategy for addressing questions about the long-term course of schizophrenia and antipsychotic drug effects on brain changes. However, the validity of our inferences depends on the observed group differences representing treatment effects rather than some systematic difference in our patient groups only indirectly related to their treatment history. In the developed world, patients who refuse treatment can be atypical. In the circumstances of the developing world, where access to services has been historically limited, economic and cultural factors and proximity to care often determine treatment access. Furthermore, the high level of psychopathology in the never-treated patient group does not suggest that they were higher functioning or had less severe psychotic disorders, allowing them to function well in the community. These considerations, together with the fact that our patient groups were recruited from the same community and did not differ in demographic factors, age at onset, or illness duration, limit to some degree concerns that our data reflect intrinsic patient group differences rather than treatment history effects. Nonetheless, these factors need to be considered when interpreting our findings.
Second, given limitations associated with our cross-sectional design, age-related modeling provides only a preliminary test for progressive brain changes. Third, as there were robust group differences across multiple tracts but differential aging effects limited to the genu, much of the change in DTI parameters appears to occur earlier than was evaluated in our study and appears not to be significantly and differentially progressive with age (5 years after onset and later). Fourth, we used a DTI protocol with 15 directions long used in our schizophrenia studies. Fifteen diffusion gradients could limit sensitivity to detect changes in the microstructural integrity of white matter fibers, especially in superficial areas. However, we note that we had sufficient power to detect significant effects in most tracts with the DTI protocol employed. Fifth, given variable drug choice and dosage over time, we were not able to link these treatment parameters to white matter alterations observed in the treated patient group. Last, to clarify mechanisms of effects, animal model work will be needed to examine longer-term antipsychotic effects on white matter to experimentally confirm a relationship between antipsychotic drugs and white matter changes, and to establish the neurobiological mechanisms underlying the relationship.
The present study revealed more widespread alteration of white matter microstructure in never-treated long-term schizophrenia patients than in those who received long-term antipsychotic treatment. These psychoradiological findings provide insights into white matter deficits over the course of schizophrenia without the confounding effects of antipsychotic medication. The results also suggest that long-term antipsychotic treatment not only does not appear to confer adverse effects on brain white matter but also may provide some benefits for white matter over the course of illness.