Tobacco use is an undisputed risk factor for increased mortality in both the general population and psychiatric patients. A meta-analysis (
1) showed that smoking is three times more prevalent among schizophrenia patients than in the general population, and adults with schizophrenia are almost 10 times more likely to die from chronic obstructive pulmonary disease compared with the general population (standardized mortality ratio=9.9, 95% CI=9.6–10.2) (
2). Although tobacco use has declined in the general population in recent decades, smoking prevalence among patients with psychosis is still high.
Cognitive deficits also occur frequently in patients with psychosis (
3). The severity and range of the affected cognitive domains in patients vary widely (
4). Cognitive domains are influenced by various underlying pharmacological mechanisms, and nicotine is known to enhance attention in patients and in the general population (
5,
6). The short-term effects of nicotine have been studied intensively in patients with schizophrenia, given that the nicotinergic acetylcholine receptor (nAChR) system that is implicated in cognitive functioning appears to be dysregulated in these patients (
7,
8). Preclinical evidence has shown that nicotine affects several neurotransmitter systems, including acetylcholine, dopamine, glutamate, and γ-aminobutyric acid (GABA), and patients with psychotic disorders may experience short-term cognitive benefits from nicotine administration (
5). However, the most recent reviews on this topic showed that nicotine or nAChR-based treatments do not enhance cognition in patients with schizophrenia (
9).
The long-term effects of smoking on cognitive functioning have been studied more extensively in the general population than in patients. A meta-analysis that included prospective studies with at least 12 months of follow-up (
10) showed that elderly smokers in the general population are at a higher risk of cognitive decline than nonsmokers. In a large cohort study (N=10,308), similar results were found for middle-aged smokers compared with nonsmokers. In addition, the risk of poor cognition was lower among people who had stopped smoking compared with current smokers (
11). Longitudinal research on the association between long-term smoking and cognition in patients with psychotic disorders, however, is scarce. Cross-sectional studies have found contradictory results (
12–
14). The largest of these studies (
14) used a composite measure of cognitive performance to assess the effect of current smoking compared with past or never smoking in patients with severe mental illness (including 400 patients with schizophrenia). Current smokers performed on a lower level overall, but results were not reported for specific cognitive domains. This is unfortunate, because among patients with psychosis, the severity and range of affected cognitive domains are generally heterogeneous (
3,
15). Moreover, a review on chronic smoking in the general population showed that cognitive domains may be differentially affected by smoking (
16).
Longitudinal evidence regarding the associations between smoking and specific cognitive functions in patients with psychosis is lacking. Moreover, it is unknown whether these associations differ in patients with psychosis compared with healthy subjects and individuals who have high vulnerability to psychosis but do not have illness-related confounders (unaffected siblings).
In this study, we examined 1) the cross-sectional association between current smoking behavior and performance in specific cognitive domains and 2) the longitudinal association between smoking behavior and cognitive functioning, as well as changes in these parameters, in a large prospective study that included patients with psychotic disorders, unaffected relatives (siblings), and healthy control subjects. We hypothesized that smoking is associated with reduced cognitive functioning compared with not smoking and that smoking cessation is associated with partial cognitive recovery in all groups.
Method
Population and Study Design
This study was performed within the naturalistic multicenter Genetic Risk and Outcome of Psychosis (GROUP) cohort study. The full sample consisted of 1,119 patients with a diagnosis within the nonaffective psychotic spectrum, 1,059 unaffected siblings, and 586 unrelated healthy control subjects. The study procedures and inclusion and exclusion criteria for participants have been described in detail elsewhere (
17). Patients diagnosed as having a nonaffective psychotic disorder according to DSM-IV criteria were recruited by clinicians from four university study sites and their surrounding mental health care facilities in the Netherlands and Belgium (
18). Trained investigators conducted clinical interviews with patients and applied rating instruments. All patients, unaffected siblings, and healthy control subjects were invited to take part in a baseline assessment and in follow-up assessments 3 and 6 years after enrollment. We analyzed all individuals for whom complete data were available for the baseline measurement of smoking in the past 12 months. The study was approved by the Medical Ethics Committee of the Academic Medical Center of Utrecht. Written informed consent was obtained from participants before they were enrolled in the study. Release 5.00 of the GROUP database was used for the analyses.
Smoking details.
The shortened version of the Composite International Diagnostic Interview (CIDI) (
19) was used to assess the quality, severity, and course of tobacco use during the past year. A special Substance Abuse Module (CIDI-SAM) covers tobacco in considerable detail, and this instrument was found to be reliable in a cross-cultural trial (
20). Participants were defined as smokers if they smoked daily during 1 month or more in the past 12 months. Data were also collected on number of cigarettes smoked per day in the period of most severe smoking in the past 12 months.
Cognitive measures.
All participants were assessed with a cognitive task battery that assessed cognitive domains comparable to those defined in the MATRICS Consensus Cognitive Battery, as described in detail previously (
3,
17). Different subtests of the WAIS-III (
21) were used to assess global cognitive functioning and to measure, specifically, the domains of processing speed (digit-symbol coding task), working memory (arithmetic), and reasoning and problem solving (block design task). A word learning task (the Auditory Verbal Learning Test [
22]) assessed verbal learning with outcome parameters of immediate recall (15-word list, three learning trials) and retention rate after 20 minutes. The Continuous Performance Test (
23) was administered to test the domain of attention/vigilance, for which the accuracy score and mean reaction time were used. The tests were administered in a fixed order. Total testing time was approximately 2 hours. A break was scheduled in the testing in case of fatigue; smokers received no special instructions regarding their smoking behavior and possibly smoked during this break.
Assessment of covariates.
Based on the well-known association with cognition and in line with previous studies (
12,
14), the following confounders were selected a priori: age, gender, years of education, cannabis use, antipsychotic medication use, and severity of psychopathology. Years of education was determined at each assessment. Cannabis use was assessed with urinalysis; urine was screened for the presence of cannabis with a 50 ng/mL THC cutoff in order to infer a detection window of 1 month. Data on antipsychotic medication use (yes/no) were collected at each assessment. Severity of psychopathology in patients was determined with the Positive and Negative Syndrome Scale (PANSS) (
24). Subclinical symptoms in siblings and control subjects were assessed with the Community Assessment of Psychic Experiences (
25).
Statistical Analysis
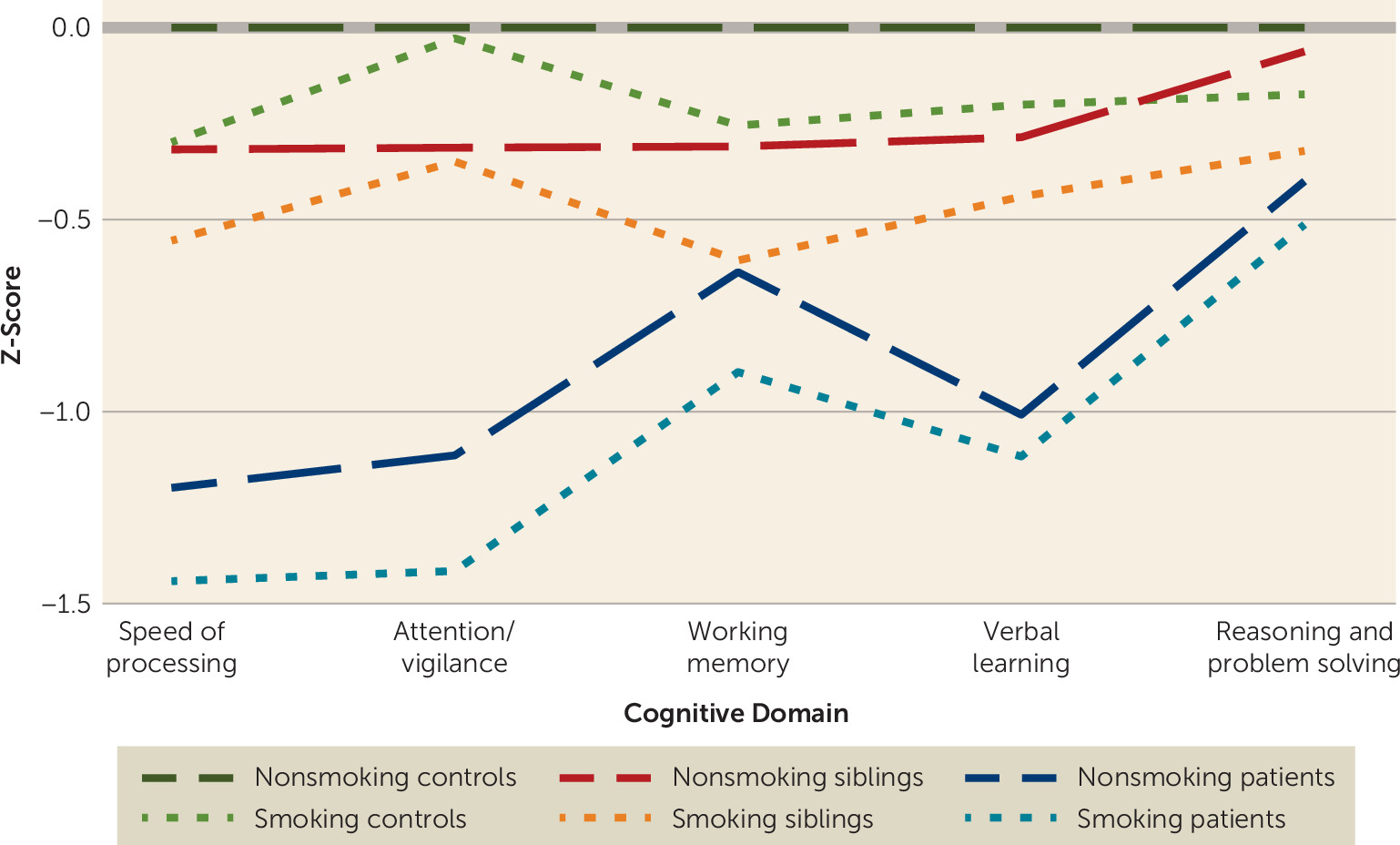
Student t tests, Pearson chi-square tests, and one-way analysis of variance were used to compare baseline differences in demographic characteristics and clinical outcomes between smokers and nonsmokers in patients, unaffected siblings, and healthy control subjects. To illustrate the baseline difference in cognitive performance, one cognitive score per domain was standardized to a z-score, using the values of the nonsmoking control subjects as reference. Next, we used R, version 3.3.2 (
26), and the lme4 package (
27) to perform linear mixed-effects analyses of the relationship between cognitive measures and smoking status over a period of 6 years. Visual inspection of residual plots revealed no obvious deviations from homoscedasticity or normality, with the exception of the accuracy score on the Continuous Performance Test. Linear mixed models were applied to the raw scores and raw change scores for each cognitive domain. In all analyses, p values were calculated by the Kenward-Roger approach, using the pbkrtest package in R (
28), which has been shown to produce the most acceptable type I error rates in mixed-effects models (
29). Given the seven outcomes in five independent cognitive tasks, we used Bonferroni correction to minimize the risk of type I errors, and thus the two-tailed significance threshold was set at 0.007 (0.05/7).
To answer the multi-cross-sectional research questions, we entered into the model, as fixed effects, smoking status during the past 12 months, time, and all confounders selected a priori. As random effects, we added intercepts for subjects and by-subject random slopes for the effect of time. Each variable was added in a forward approach, and the Akaike information criterion was used to compare model fit. A lower Akaike information criterion estimate for the model after adding the covariates indicates a better model fit. Post hoc linear mixed-model analyses were conducted if a significant difference was found between smokers and nonsmokers for a specific task. To specifically investigate the association of cognition with smoking severity, we then entered the number of cigarettes per day instead of smoking status and subsequently entered the same fixed and random effects as stated above.
To answer the research questions concerning the effect of change in smoking behavior, we determined change in smoking status at 3-year follow-up compared with baseline and at 6-year follow-up compared with 3-year follow-up. In a similar fashion, a change score was calculated for each period for number of cigarettes per day and raw scores for each cognitive task. Next, we used linear mixed models with the same a priori covariates to explore differences in change scores of cognitive performance between subjects who quit smoking, those who started smoking, and those whose smoking status did not change. The same set of fixed and random effects as used to answer the first research question were entered. If a significant result was found, we conducted post hoc analyses to examine the relationship between change in number of cigarettes per day and cognitive performance with the same set of fixed and random effects.
Results
Study Sample Characteristics
In total, we analyzed data from 1,094 patients with a nonaffective psychosis, 1,047 siblings, and 579 control subjects at baseline. The demographic and clinical characteristics of smokers and nonsmokers are presented in
Table 1. A total of 717 (65.5%) patients were diagnosed as having schizophrenia (DSM-IV codes 295.1, 295.2, 295.3, 295.6, and 295.9). Baseline smoking rates were significantly higher in patients compared with siblings and compared with healthy control subjects (66.6%, 38.3%, and 25.2%, respectively; χ
2=312.49, p<0.001). Patients who smoked also used significantly more cigarettes per day (mean=17.8, SD=8.6) than did smoking siblings (mean=13.1, SD=9.1) or control subjects (mean=12.0, SD=7.7) (F=60.28, p<0.001). Smoking patients, siblings, and control subjects showed significant differences in various demographic characteristics and in the presence and severity of psychopathology compared with nonsmokers (
Table 1). Unadjusted baseline cognitive scores were lowest in the patient group and highest in the nonsmoking control group (
Figure 1).
Multi-Cross-Sectional Association Between Smoking and Cognitive Performance
To explore the overall association between smoking status and cognitive outcomes over 6 years, we used data from 916 patients, 947 siblings, and 552 control subjects (
Table 2; see also section 1 in the
online supplement). Data on cognitive performance scores were missing for a maximum of 179 patients, 100 siblings, and 27 control subjects. In patients, mixed-effects analyses revealed a significant negative association between smoking and score on the digit-symbol coding task (estimate=−2.38, SE=0.84, p=0.005). Post hoc analysis (see section 2 in the
online supplement) revealed a similar negative association between number of cigarettes per day and score on the digit-symbol coding task (estimate=−0.10, SE=0.03, p=0.002). In siblings, significant negative associations were found between smoking and score on the arithmetic task and the block design task (
Table 2). Post hoc analyses also showed negative associations between number of cigarettes per day and score on the block design task (estimate=−0.13, SE=0.04, p=0.002). In control subjects, we found a significant negative association between smoking and score on the digit-symbol coding task (
Table 2). Finally, analyses yielded a negative association between number of cigarettes per day and score on the digit-symbol coding task (estimate=−0.25, SE=0.07, p=0.001). We did not find significant associations between smoking status and performance on the Auditory Verbal Learning Test and the Continuous Performance Test in any of the three groups.
Prospective Association Between Change in Smoking Behavior and Cognitive Performance
Overall, patients, siblings, and control subjects showed significantly better performance on most cognitive tasks during follow-up (see sections 3 and 4 in the online supplement). We found that smoking behavior remained unchanged in most individuals from one assessment to the next (patients, 89.6%; siblings, 86.1%; control subjects, 90.7%). Over time, however, more individuals quit smoking (patients, 6.7%; siblings, 7.8%; control subjects, 5.4%) than started smoking (patients, 3.7%; siblings, 6.1%; control subjects, 3.9%).
We found a significant positive association between smoking cessation and change scores on the digit-symbol coding task (estimate=4.90, SE=1.73, p=0.005), indicating that processing speed improved in patients who quit smoking compared with patients who did not change their smoking behavior (see section 3 in the online supplement). This association was not found in siblings and control subjects. No significant associations were found between smoking initiation and changes in cognitive functions in any of the groups (see section 3 in the online supplement). In the patient group, post hoc analyses revealed a significant negative association between change in number of cigarettes per day and change in score on the digit-symbol coding task (estimate=−0.18, SE=0.05, p=0.001).
Discussion
As in most other studies, the prevalence of smoking and the number of cigarettes smoked per day were much higher among patients with psychotic disorders than among unaffected siblings and control subjects. Consistent with our hypothesis, smoking was associated with lower performance in cognitive functions (e.g., processing speed, working memory, and reasoning and problem solving) in all groups—patients, unaffected siblings, and control subjects. In addition, a dose-response relationship was found for the multi-cross-sectional association of number of cigarettes per day with processing speed and reasoning and problem solving. Notably, we found a positive association between smoking cessation and processing speed only in the patient group. These findings provide new insights on the relationship between smoking behavior and cognitive functioning in patients with psychotic disorders, suggesting that smoking cessation or reduced smoking may improve processing speed in this population.
Multi-Cross-Sectional Association Between Smoking and Cognitive Performance
Previous studies on smoking and cognition in patients with psychotic disorders have reported contradictory findings. Our results are in line with the largest cross-sectional study so far, which reported reduced functioning in smoking patients compared with nonsmoking patients with psychotic disorders, based on a cognitive composite score in a total of 400 patients (
14). In two other cross-sectional studies (
12,
30) that included patients with first-episode psychosis (N=304 and N=159), no differences were found between smokers and nonsmokers on specific cognitive tasks. One small study (
13) found an advantage of smoking (N=32) compared with nonsmoking (N=15) for verbal memory outcomes in patients with schizophrenia. However, research in patients with psychotic disorders has varied in sample characteristics, with differences in illness duration, sample size, and adjustment for covariates. Our study also adds relevant findings from analyses of number of cigarettes smoked per day, showing a dose-response relationship between number of cigarettes and impairments in processing speed in patients and control subjects, and in reasoning and problem solving in siblings.
Because cognitive tasks that assess working memory and problem solving also impose a time limit, processing speed could represent a core cognitive symptom that is negatively associated with smoking. This hypothesis is consistent with the already mentioned negative association between smoking and a cognitive composite score in patients with psychotic disorders (
14) and with previous studies showing that overall cognitive performance in patients with schizophrenia can be explained by one component or a single factor based on five domains (
31,
32). Although we did not test this in the present study, the lower performance in processing speed in smoking patients could represent worse cognitive performance overall. Finally, in studies in the general population, chronic cigarette smoking has repeatedly been found to be associated with lower cognitive functioning in multiple domains beyond processing speed (
16,
33). Large studies, including the present study, point to a negative association between smoking and cognitive functioning for both patients and individuals without psychosis.
Lower cognitive performance in smokers probably reflects a multifactorial etiology, with a large number of directly cytotoxic compounds from cigarettes (e.g., carbon monoxide, polycyclic aromatic hydrocarbons) causing adverse effects in the brain (
16). Despite the large body of evidence regarding smoking and general health problems, studies concurrently assessing cognitive, neurobiological, and genetic factors in relation to chronic smoking are still scarce. A review of this issue in the general population (
16) that summarized a limited number of studies stated that chronic smoking is related to globally decreased brain perfusion, an abnormal increase in global brain atrophy, and structural and biochemical abnormalities in the anterior frontal region, subcortical nuclei, and commissural white matter.
Prospective Association Between Change in Smoking Behavior and Cognitive Performance
A study on the short-term effects of nonsmoking on cognitive performance in 26 patients with schizophrenia (
34) found no significant effect on any cognitive task after 3 weeks of smoking abstinence. To our knowledge, the present study is the first to find a significant positive association between smoking cessation and processing speed in patients with psychotic disorders. We have not found any studies on the association between sustained tobacco abstinence and cognitive performance in patients with psychosis. A meta-analysis of abstinence from various kinds of substances, but not tobacco, found that abstinence generally results in partial cognitive recovery (
35). There are currently no published studies in patients or the general population that evaluated whether this positive association can be explained by neurobiological processes, such as brain plasticity (e.g., capacity to change in response to smoking cessation) and perfusion rates. Nevertheless, the accumulating evidence concerning the neurotoxic effect of persistent smoking is substantial. In the general population, it has been found repeatedly that former smokers showed less cognitive decline than those who continued smoking in later life (
11,
36). Moreover, more lifetime years of smoking has been found to be related to poorer performance on measures of cognitive efficiency, processing speed, and visuospatial skills (
33). Research relating measures of cognitive function to neurobiological integrity is therefore needed to understand whether or to what extent the harm of smoking is reversible in patients with psychotic disorders.
Strengths and Limitations
The main strengths of this study are the large sample size, the presence of two comparison groups, and the prospective long-term nature of the study, with follow-up assessments after 3 and 6 years. The study also has some limitations. First, given the observational design, no definite causal conclusions can be drawn, since reverse causation cannot be ruled out (e.g., patients who show improvement in cognitive performance also stop smoking). Second, and related to the first limitation, this study focused on spontaneous changes in smoking status and their relation to cognitive functions. Lower cognitive functioning is a known vulnerability for relapse of substance abuse (
37), and patients who quit smoking could therefore represent a subgroup that had a larger increase in cognitive performance than those who did not change their smoking behavior. Third, information about exposure to smoking was limited. Observation points within the GROUP study have a 3-year interval, and participants were interviewed regarding their smoking behavior only over the 12 months before each assessment. Smoking history (i.e., number of pack-years) was not assessed, and this barred correction for length of exposure. This is of interest because a greater number of lifetime years of smoking has been found to be related to poorer performance on measures of cognitive efficiency, processing speed, and visuospatial skills (
36). Fourth, no data were available on blood levels of cotinine or carbon monoxide. However, in a systematic review and meta-analysis about the validity of self-reported smoking behavior (
38), interviewer-administered questionnaires, such as the CIDI-SAM, which was used in this study, were shown to produce accurate data. Fifth, GROUP patients represent a relatively high-functioning cohort, probably with higher cognitive performance than average patients with psychosis, which limits the generalizability of our findings. Finally, a minor limitation was the use of accuracy scores from the Continuous Performance Test to assess sustained vigilance. This measure showed ceiling effects, not reacting to transformation. By also including the mean reaction time scores from the Continuous Performance Test, we aimed to bypass assessment of nondiscriminating values.
Implications
We found substantially higher smoking rates in patients with psychotic disorders compared with unaffected siblings and control subjects, and lower cognitive performance in several domains in smokers compared with nonsmokers, irrespective of their illness or vulnerability status. Two main hypotheses have been proposed to explain the high smoking rates in patients with schizophrenia: the self-medication hypothesis and the shared-vulnerability hypothesis (
7). The self-medication hypothesis postulates that patients often smoke to alleviate clinical symptoms, such as cognitive impairments. This theory is criticized by researchers who believe that shared genetic and shared environmental factors together with neurological deficits make individuals with schizophrenia more vulnerable to tobacco use and nicotine dependence (
39,
40). Our prospective results on the cognitive effects of changes in smoking status in patients with nonaffective psychosis do not support the self-medication hypothesis. This is illustrated by our finding that smoking in patients with psychotic disorders is associated with impairment in domains similar to those observed in smoking nonpatients (after correcting for crucial confounders such as variation in psychopathology) and the fact that patients who quit smoking showed a significant improvement in processing speed. Moreover, our finding that smoking is more prevalent in both patients and unaffected siblings of patients (i.e., siblings without illness-related confounders) compared with healthy control subjects points to the presence of shared genetic or environmental factors that increase the risk of both smoking and psychosis. Still, patients often report that they smoke because of a need for stimulation and activation (
41). We argue that more psychoeducation about the potential long-term negative effects of smoking on cognitive performance is necessary. The results of our study further underline the importance of smoking cessation treatment to increase cognitive, mental, and somatic health in patients with psychosis.
Acknowledgments
The authors thank all the research personnel involved in the GROUP project, and in particular Joyce van Baaren, Erwin Veermans, Ger Driessen, Truda Driesen, Karin Pos, Erna van ’t Hag, Jessica de Nijs, Atiqul Islam, Wendy Beuken, and Debora Op ’t Eijnde. They also thank the participating patients, their families, and the healthy subjects for their time and effort.