Impaired cognition is a hallmark of schizophrenia (
1): deficits are pervasive, occurring in 75%−85% of patients (
2), and frequently precede the onset of other symptoms (
2,
3). These deficits also are persistent (
4), remaining after psychotic symptoms have been effectively treated (
5). Cognitive deficits are among the best predictors of certain aspects of functional outcome in schizophrenia (
6), and their presence predicts reduced adaptive and social skills (
7) and increased risk of relapse in first-episode patients (
8). The impacts of cognitive deficits on everyday functioning appear to be mediated by their association with the patient’s ability to perform everyday functional skills, which in turn predicts everyday disability (
9). Impairments are also found in first-degree relatives of patients and individuals with prodromal symptoms (
4).
Cognitive deficits in domains impaired in schizophrenia, including attention, working memory, episodic memory, and executive functioning, have been identified in a similar pattern in people with other schizophrenia spectrum disorders, such as schizotypal personality disorder (
10) and attenuated psychosis syndrome. Patients with schizotypal personality disorder exhibit deficits in the same domains as people with schizophrenia, with impairments in verbal and spatial episodic memory, vigilance, attention, abstract reasoning, recognition memory, cognitive inhibition, verbal fluency, and verbal and spatial working memory (
11). Studies of cognition in schizotypal personality disorder avoid confounders associated with antipsychotic medication treatment, and the cognitive deficits of schizotypal personality disorder are less global than in schizophrenia, providing a unique opportunity to test new interventions for cognitive enhancement in schizophrenia spectrum disorders.
Emerging research suggests that individuals with schizotypal personality disorder demonstrate impaired performance on measures of functional capacity similar to that found in schizophrenia (
11), as well as manifesting similar impairments in everyday functioning (
12). In fact, in a study of cognitive deficits, functional capacity (as assessed by the University of California San Diego Performance-Based Skills Assessment [UPSA]), and real world-functioning in schizotypal personality disorder (
11), we found that the correlation between functional capacity and cognition in schizotypal personality disorder was as strong as that seen in multiple previous studies of schizophrenia. Patients with schizotypal personality disorder had reduced educational attainment, earned less money per hour, and were less likely to live independently compared with healthy control participants or participants with avoidant personality disorder. Thus, treatment of cognitive deficits in schizotypal personality disorder not only can serve as a model for treatment efforts in schizophrenia but also is important because real-world functional gains and possible improvement in functional capacity may be realized in patients with schizotypal personality disorder.
Cognitive remediation therapies have demonstrated efficacy for improving cognition in schizophrenia (
13). These approaches, using techniques from clinical neuropsychology, focus on the acquisition of cognitive skills in the areas most impaired in schizophrenia, such as attention and concentration, psychomotor speed, learning and memory, and executive functions (
14). These programs generally use computerized strategies that use adaptive titration of difficulty to allow patients to move more quickly through areas in which they have better skills and to spend more time on areas of greater impairment. Cognitive remediation has been shown to have substantial success when psychiatric rehabilitation interventions targeting skills deficits, such as supportive employment services, are added to the cognitive remediation (
15). In addition, there is evidence to support the effectiveness of cognitive remediation for patients on the broader spectrum, for example for attenuated psychosis (
16,
17).
Although cognitive remediation has been shown to effectively ameliorate cognitive deficits in patients with schizophrenia, trials of pharmacological agents targeting these symptoms have been much less successful. Recent research, however, suggests that there may be a detectable benefit from synergistic approaches that combine pharmacological interventions with cognitive remediation or psychosocial interventions (
18). To our knowledge, no one has examined the triple interaction of pharmacological interventions, computerized cognitive remediation, and a psychosocial intervention such as social skills training, which may offer the most benefit to patients in terms of improved cognition and functional outcome.
One agent with some support for augmenting cognitive remediation and social skills training is guanfacine, a selective agonist for the adrenergic α
2a-receptor subtype (
19). Alpha
2A-adrenergic agonists improve cognitive function in a variety of disorders, such as attention deficit hyperactivity disorder (
20), Tourette’s syndrome (
21), opioid dependence (
22), and pervasive developmental disorder (
23). Treatment with guanfacine increases cerebral blood flow in the anterior frontal cortex in regions critical to working memory performance (
24). Furthermore, participants with schizotypal personality disorder treated with guanfacine demonstrated improvement on a context processing task, which is heavily dependent on working memory, compared with those given a placebo (
19). Additionally, schizophrenia patients treated with guanfacine alone have shown some small improvements (
25).
In this study, we sought to extend the literature in two important ways. First, although individuals with schizotypal personality disorder demonstrate similar, albeit attenuated, cognitive deficits compared with individuals with schizophrenia, to our knowledge, there have been no studies to date of cognitive remediation as a treatment for cognitive deficits found in schizotypal personality disorder. Second, although several studies have investigated the possible pharmacological facilitation of a cognitive remediation therapy regimen in individuals with schizophrenia (
18), the pharmacological studies in schizotypal personality disorder have examined medications alone. Thus, the present study is the first, to our knowledge, to investigate the synergistic effect of a pharmacological intervention, cognitive remediation, and a psychosocial treatment.
Our goal in this study was therefore to evaluate the ability of guanfacine to augment cognitive remediation and social skills training in individuals with schizotypal personality disorder, in a double-blind, placebo-controlled trial. We hypothesized that cognitive remediation and social skills training would improve cognitive performance and functional skills and that participants with schizotypal personality disorder assigned to receive guanfacine treatment would exhibit greater improvement than those treated with cognitive remediation and social skills training alone.
Methods
Participants
Participants were recruited from the community in and around the Icahn School of Medicine at Mt. Sinai in New York City, via newspaper and online advertisements targeting key features of schizotypal personality disorder (magical thinking, odd beliefs, and social isolation). Throughout the enrollment period, 744 individuals responded to these ads by calling the research office. Of these, 104 declined to schedule a screening interview, 345 failed to attend their screening interview, and 136 were excluded after the screening interview because they met one or more exclusionary criteria for the study. An additional 123 participants were excluded after failing to meet diagnostic criteria for schizotypal personality disorder or failing the general medical clearance, and eight participants who met eligibility criteria declined to participate because of scheduling conflicts or lack of interest in medication, leaving 28 individuals (21 men, seven women) with DSM-IV-defined schizotypal personality disorder who enrolled in the trial. Participants ranged in age from 22 to 62 years (mean=43.78, SD=11.44). All participants were free of psychotropic medication at the time of enrollment and were medically healthy, as determined by a full medical clearance including ECG, medical history, physical examination, and laboratory tests. Participants with a lifetime diagnosis of a psychotic disorder (schizophrenia, schizoaffective disorder, or bipolar disorder I disorder with psychotic features) or current major depressive episode were excluded, as were participants with current substance abuse or lifetime substance dependence. Participants were evaluated using the Structured Interview for DSM-IV Personality Disorders (
26) and the Structured Clinical Interview for DSM-IV Axis I Disorders (
27). All interviews were conducted by doctoral-level clinical psychologists. Diagnoses were reached by consensus in a meeting of all raters with an expert diagnostician. Although none of the participants met diagnostic criteria for any DSM-IV psychotic disorder, subclinical psychotic symptoms, consistent with a diagnosis of schizotypal personality disorder, were prevalent in this sample. Seventy-one percent of participants endorsed ideas of reference, 57% reported odd beliefs or magical thinking, 57% endorsed unusual perceptual experiences, 82% demonstrated odd thinking and speech, 79% endorsed paranoid ideation, and 64% exhibited odd appearance.
All participants provided informed consent in accordance with the Institutional Review Board of the Icahn School of Medicine at Mount Sinai.
Materials
MATRICS Consensus Cognitive Battery.
The MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) Consensus Cognitive Battery (MCCB) (
28) was developed as a primary outcome measure for clinical trials of cognitive enhancement in schizophrenia and has become the gold standard in this field. For this trial, five MCCB domains, all of which have been implicated in schizophrenia patients, were included: speed of processing, working memory, verbal learning, visual-spatial learning, and reasoning and problem solving (social cognition was assessed using a supplemental measure in this study). For all MCCB domains, age-corrected T-scores served as the dependent variable.
UCSD Performance-Based Skills Assessment (UPSA).
The UPSA (
29) is an office-based test to assess the ability of schizophrenia patients to perform daily tasks. This test was designed for outpatients and measures performance in several domains of everyday functioning using props and standardized performance situations. In line with our previous research (
30–
32), four domains of the UPSA were assessed: comprehension/planning, finance, communication, and transportation/mobility. Lower scores on the UPSA have been demonstrated to correlate with more severe cognitive deficits and negative symptoms, but not with severity of positive psychotic symptoms, in people with schizophrenia (
6). UPSA scores also have been demonstrated to correlate with cognitive performance and real-world functioning in people with schizotypal personality disorder (
11). The UPSA total score summed across the four domains served as the dependent variable for the present study.
Movie for the Assessment of Social Cognition (MASC).
The MASC (
33) is a 15-minute movie about four characters getting together for a dinner party. Administration of this assessment involves pausing the video 45 times and asking the patient questions concerning the characters’ feelings, thoughts, and intentions. The assessment takes 40 minutes to complete. The multiple-choice version of the MASC allows for analysis of qualitative social cognition errors. The MASC dependent variables consist of mentalization accuracy, summed score for all correct questions; hypomentalization errors (hypomentalization or no mentalization); and hypermentalization errors. For the present trial, hypomentalizing errors, which are more closely associated with cognitive and negative symptoms in schizophrenia, served as the dependent variable (
34).
Additional neuropsychological assessments.
We supplemented the MCCB with four neuropsychological assessments of working memory and context processing. The N-back working memory task is a commonly used measure of working memory (
35,
36). Participants observed letters presented on a computer screen one at a time over three conditions: 0-back, 1-back, and 2-back. In the 0-back condition, participants responded to a single prespecified target letter (e.g., X). In the 1-back condition, the target was any letter identical to the one immediately preceding it (i.e., one trial back). In the 2-back condition, the target was any letter identical to the one presented two trials back. For this study, the dependent variables were the number of correct responses for the 2-back condition. The Paced Auditory Serial Addition Test is a test of auditory verbal working memory that has been well described and validated in schizophrenia and schizotypal personality disorder samples (
37,
38). Participants listened to a tape-recorded voice presenting a series of numbers (50 numbers at a rate of one digit per 2 seconds) and were asked to add each adjacent pair of numbers and respond by verbalizing the sum. The total number of correct responses was the dependent variable. The Dot Test (
39) is a test of visuospatial working memory in wide research use. Participants were presented a dot at a specific position on a standard-sized sheet of paper and were then asked to reproduce it at the same location on a separate sheet after different periods of delay (no delay and 10-, 20-, or 30-second delays). Performance was measured as the distance in centimeters between the drawn dot and the actual dot (distance error). The distance error at the 30-second delay (longest memory load of all three delays) minus the distance error at the immediate condition was the dependent variable. During the AX-Continuous Performance Test, sequences of letters were visually presented one at a time in a continuous fashion on a computer display. Participants were instructed to make an affirmative response on target trials and a negative response otherwise (
40). The delay between cue and probe was 5 seconds, and the intertrial interval was 1 second. The task was presented in two blocks of 50 trials. Participants were asked to respond as quickly as possible to each stimulus while maintaining accuracy. The d′-context score served as the dependent variable.
Cognitive remediation therapy intervention.
We used a multimedia (auditory and visual) Windows-based program designed by Psychological Services, Inc. (
41) for cognitive remediation therapy. The exercises in the program are aimed at improving areas of deficit within the schizophrenia spectrum. We chose this software because it has been successfully paired with social skills training and has sophisticated graphics that we hoped would appeal to a community-recruited sample. In each session of every cohort, participants were divided into groups of two to three participants and engaged in the exercises; participants worked independently on individual laptop computers but were encouraged to interact with one another to stimulate problem-solving skills, as well as to provide opportunities to improve social skills. Each group was led by a bachelor’s- or master’s-level research assistant who had received training to operate the audiovisual equipment and was familiar with each of the exercises. The primary role of the facilitator was to ensure that computers and software were operating properly, to gently encourage participants to stay on task and interact when opportunities presented themselves, and to monitor the number of correct responses for each participant. Although not present during computer sessions, a trained doctoral-level psychologist knowledgeable about cognitive remediation was available for consultation as needed. For this study, cognitive remediation therapy consisted of group members first working on exercises to increase attentional functioning and then, after two sessions, selecting any of the eight memory exercises or six problem-solving exercises at subsequent sessions. Each exercise provided graduated difficulty levels and other modifiable parameters. The group facilitator encouraged participants to challenge themselves, monitored their performance, and manually adjusted the difficulty of each exercise to attain an approximately 80% correct response rate for each participant.
Social skills training.
The twice-weekly social skills training followed the manualized curriculum outline and materials provided from the cognitive enhancement therapy program (
42). This manualized program was specifically designed to augment training with Psychological Services, Inc., software. Although we made substantial modifications to the full cognitive enhancement therapy program for the present study, the basic design, structure, and execution of the program were maintained. Similar to the full program, the present study incorporated the core material from each of the three modules. The first module provides participants with an orientation and overview of basic concepts. The second module consists of social cognitive skills training. The final module focuses on real-world applications of cognitive and social cognitive skills acquired during the therapy. All groups were led by a doctoral-level clinical psychologist. Participants received a small monetary compensation for attendance at each group session.
Procedure
The baseline cognitive and functional skill assessment battery was completed prior to initiation of guanfacine or placebo. After completion of the assessment battery, participants entered an 8-week, double-blind, placebo-controlled treatment phase during which they were randomly assigned to receive guanfacine or placebo. All participants with schizotypal personality disorder recruited for this trial performed at least one standard deviation below the mean of the healthy control participants on at least one MCCB domain. Participants taking guanfacine were titrated to 2.0 mg/day during the first 2 weeks and remained on 2.0 mg/day for the duration of the study. Participants’ blood pressure and heart rate were measured weekly, and participants met with the study physician weekly to assess for potential side effects. Fifteen participants were randomly assigned to receive guanfacine, and 13 to receive placebo. At the completion of the trial, medication was discontinued for all participants, although those who reported that they experienced benefit from the medication were encouraged to follow up with their medical providers.
All participants received the active cognitive remediation and social skills training intervention, which consisted of a combination of computer-based cognitive enhancement exercises (
43) and manualized social skills training modified from cognitive enhancement therapy (
42). Specifically, participants met for 60 minutes of computer-based cognitive enhancement exercises and 60 minutes of social skills training twice weekly (4 hours total per week) over 8 weeks, for a total of 30 sessions (15 sessions of computer-based cognitive remediation and 15 sessions of small group social skills training).
The cognitive and functional skill assessment battery was readministered at the completion of the trial.
Data Analysis
Data were analyzed using SPSS 21.0 for Mac. The primary endpoint was cognition as evaluated by the MCCB domains. Secondary endpoints were social cognition, as evaluated by the MASC, and function skills, as evaluated by the UPSA. We computed a series of 2 (time: baseline, posttreatment) by 2 (treatment condition: guanfacine, placebo) repeated-measures analyses of variance (ANOVA), one for each MCCB domain, as well as for UPSA total score and MASC hypomentalizing errors.
Results
In the guanfacine group, 11 participants were male and four female, nine were right-handed, and the mean age was 46.5 years (SD=12.2), and in the placebo group, 10 participants were male and three female, 10 were right-handed, and the mean age was 40.7 years (SD=10.1); there were no significant differences between groups on these measures. Adherence to the protocol was excellent. All participants completed the trial except for one participant who was removed from the trial because of an increase in blood pressure noted at the weekly medical check-in, although this participant was later revealed to have been assigned to the placebo group. Across both the computerized cognitive remediation and group social skills training sessions, mean attendance was 80%. In addition, guanfacine was well tolerated by this population, with no side effects noted in the active treatment group.
Results of the repeated-measures ANOVAs suggested that participants with schizotypal personality disorder benefited from the cognitive remediation and social skills training (
Table 1). We found statistically significant main effects for time (pretreatment compared with posttreatment) on MCCB speed of processing (F=6.86, df=1, 24, p=0.015), verbal learning (F=5.14, df=1, 25, p=0.011), and visual learning (F=7.50, df=1, 25, p=0.032). A statistically significant improvement was observed across groups for UPSA total score (F=5.73, df=1, 24, p=0.025). For all variables, participant performance improved after the intervention (
Table 1).
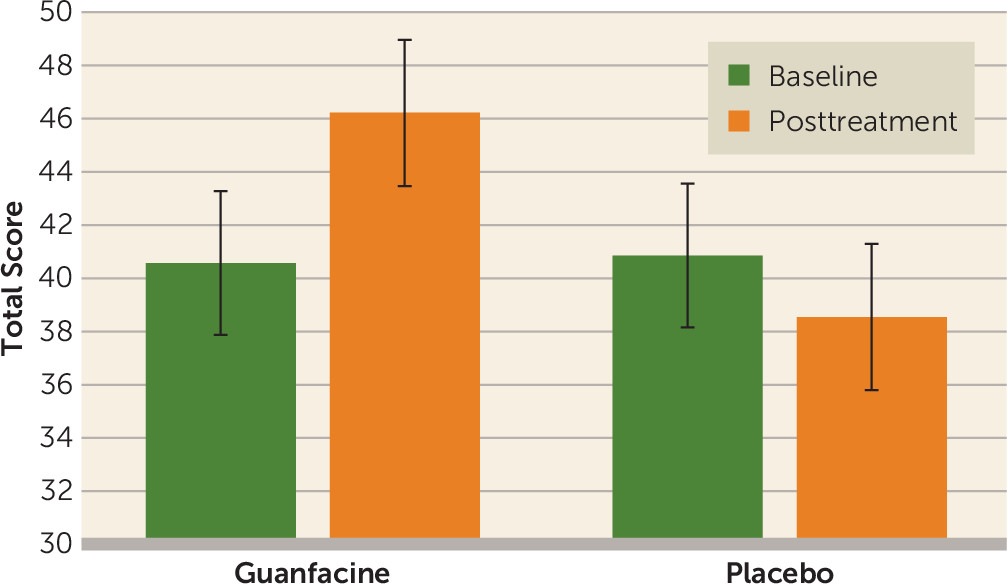
In addition, a significant time-by-medication interaction was observed for the MCCB reasoning and problem-solving domain (F=8.36, df=1, 25, p=0.005;
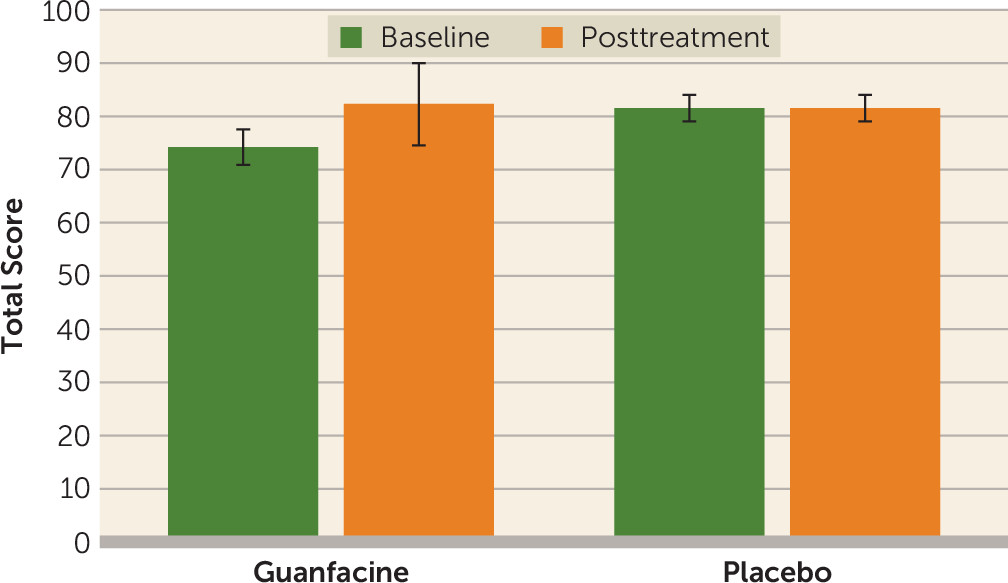
Figure 1) and for UPSA total score (F=5.62, df=1, 24, p=0.026;
Figure 2), with individuals in the guanfacine group demonstrating greater improvement over the course of treatment than those in the placebo group on both cognition and functional skills. Finally, the time-by-medication interaction for MASC hypomentalization errors approached statistical significance (F=3.55, df=1, 22, p=0.07). No statistically significant change was observed in MCCB working memory or in the supplemental neuropsychological assessments (all p values >0.05) (see the
online supplement).
Discussion
Overall, we found that the intervention was well tolerated by participants with schizotypal personality disorder. Adherence to the cognitive remediation and social skills training protocol was excellent, and no side effects were noted for the medication, guanfacine. Individuals with schizotypal personality disorder appeared to benefit from the cognitive remediation and social skills training. Statistically significant improvements were seen in three domains of cognitive performance—speed of processing, verbal learning, and visual learning—after 8 weeks of cognitive remediation and social skills training. Because two of the three domains were also assessed by alternate forms of memory tests, practice effects would be expected to be minimized. In addition, participants with schizotypal personality disorder demonstrated improved scores on the UPSA after the cognitive remediation and social skills training.
Most importantly, guanfacine augmentation enhanced the impact of cognitive remediation and social skills training in this sample. Individuals who were given guanfacine in conjunction with cognitive remediation and social skills training demonstrated significantly greater improvement in their reasoning, problem-solving, and functional skills, and they demonstrated improvements in social cognition that approached statistical significance. Together, these results suggest that this agent, which is hypothesized to enhance an individual’s attentional ability, augments the results of cognitive remediation and social skills training.
During computer sessions, participants were encouraged to problem solve together and support each other. The computer training was thus an additional forum in which to practice skills discussed during the social cognition training. Some members were interested in continuing connections with other group members and exchanged contact information for subsequent meet-ups outside the group. This behavior was neither encouraged nor discouraged by group facilitators. For some of the participants, the group content and computer training provided a shared experience and common ground, while others reported less interest in making connections and a greater focus on improving task performance. In general, although symptoms of schizotypal personality disorders remained present for most participants at the conclusion of the trial, the majority demonstrated improvements in cognitive performance, functional skills, and social skills and reported qualitative benefit from the experience.
Our study included several innovations. To our knowledge, this is the first study to examine the efficacy of a combined psychosocial intervention—cognitive remediation and social skills training—in a sample of individuals with schizotypal personality disorder, a schizophrenia spectrum disorder in which there is considerable functional impairment but no efficacious treatment. Furthermore, it is the first study to examine the synergistic impact of computerized cognitive remediation, a psychosocial intervention, and the pharmacological agent guanfacine. Although cognitive remediation trials in schizophrenia have demonstrated considerable benefit, not all participants respond or respond equally well to such interventions. Identification of agents and adjunctive therapies that augment cognitive remediation is therefore an important step in increasing the effectiveness of the intervention.
This study has several limitations. There was no inactive treatment; all participants received the combined psychosocial intervention. Thus, a placebo effect cannot be excluded as an explanation for the participant improvements from baseline. As noted above, some of the skills that improved were assessed with alternate forms; however, this limitation does not apply to the differences in improvements seen between the guanfacine and placebo groups in this study. Our sample size was small, and it is difficult to determine how representative our patients were compared with patients in other studies of schizotypal personality disorder, which also have had small sample sizes. Finally, practice effects are always a potential explanation for improvements in performance after retesting. In our recent study (
44) addressing practice effects on the MCCB in patients with schizophrenia, we found that, on average, MCCB scores improved by a little more than 1 point per reassessment. Because our statistically significant changes on the MCCB were considerably larger than that, we assume that practice effects are not a viable explanation for changes in performance in the group that received cognitive remediation and social skills training alone in the present study.
The results of our study suggest that cognitive remediation and social skills training are an effective intervention for improving cognitive performance and functional skills in individuals with schizophrenia spectrum disorders and that guanfacine is a promising agent for enhancing the effectiveness of the intervention. Because cognitive impairments are closely linked to functional outcomes for individuals across the schizophrenia spectrum, this augmented therapy is an important next step in improving real-world outcomes for individuals with these disorders.
Acknowledgments
Supported by a NARSAD Young Investigator Award from the Brain and Behavior Foundation to Dr. McClure; by NIMH grant MH 097799 to Dr. New; by VA Merit Award i01CX000609-01 to Dr. Hazlett; and by the VA Advanced Fellowship in Mental Health, VISN2 Mental Illness Research, Education, and Clinical Center.