Functional impairments persist in schizophrenia spectrum disorders despite adequate reduction of psychotic symptoms with pharmacological and psychosocial intervention (
1). Recovery of community functioning is a primary goal for people with schizophrenia and is associated with better quality of life and fewer treatment-associated costs (
2). Yet, only 25% of individuals with schizophrenia experience functional recovery (
3). Neurocognitive impairments, associated with reduced activation of lower-order (mismatch negativity, P300 task) and higher-order (theta synchronization) EEG markers, are persistent features of schizophrenia consistently found to be more closely associated with functional outcomes than clinical symptoms are (
4). Cognitive remediation is a psychological intervention designed to enhance neurocognitive abilities, with the goal of improving community functioning.
Two meta-analyses suggest that cognitive remediation produces moderate improvements in neurocognition; however, improvements in community functioning are inconsistent (
5,
6), which may be partly a result of the specificity of the targeted cognitive domain (for a review, see reference
7). The two most common training targets in cognitive remediation are perceptual abilities and higher-order cognitive functions.
Theoretical support for perceptual training comes from evidence that disturbances in the earliest stages of perception in schizophrenia cause dysfunction in higher-order domains, such as verbal memory (
8), social cognition (
9), and community functioning (
9). If perceptual input can be made less noisy, then higher-order cognitive operations should function more effectively (
10). Perceptual training directly trains these impaired perceptual abilities and has produced improvements in oscillatory brain activity (
11), auditory processing (
12), verbal memory (
13), and global neurocognition (
13). Despite these promising improvements on proximal treatment targets, there is less evidence for generalization to higher-order cognitive domains or community functioning (
14).
Theoretical support for executive training comes from findings that executive control impairments are a core feature of schizophrenia. Individuals with schizophrenia have inefficient distribution of executive network processing (
15) and reduced synchronization of frontal neural networks during executive function tasks (
16). Executive training directly trains higher-order cognitive abilities and has been found to increase gray matter in frontal cortical areas (
17), enhance frontal neural synchronization (
18), and increase activation of the central executive network (
19). Executive training improves higher-order cognitive skills, such as cognitive flexibility and working memory (
20), and there is evidence that social functioning and self-esteem improve when these higher-order skills are the treatment target (
20).
Despite evidence supporting differential effects from both approaches, comparisons of perceptual training and executive training are not found in the literature. In this study, we compared the effects of perceptual training and executive training on neurophysiological measures, neurocognition, functional competence, and real-world functioning. Given the greater functional relevance of executive skills and strategies and early evidence that executive training can improve social functioning (
20), we hypothesized that executive training would produce larger and more durable effects on neurocognition, functional competence, and real-world functioning than perceptual training. We further hypothesized that executive training would produce larger effects on higher-order EEG measures, whereas perceptual training would produce larger effects on lower-order EEG measures.
Methods
Study Design and Participants
This was a double-blind (participant, outcome assessor) parallel randomized controlled trial with equal allocation to perceptual training and executive training.
Eligible individuals between ages 18 and 65 with a diagnosis of a schizophrenia spectrum disorder were recruited from three mental health outpatient services in Kingston, Ontario. Exclusion criteria included active substance abuse within the past 12 months; history of acquired brain injury, developmental disability, or neurocognitive disorder; and cognitive remediation within the past 6 months. Participants had to be able to speak English.
Interventions
The perceptual training and executive training interventions were structurally identical and consisted of a 6-week treatment window with two therapist-facilitated 1-hour sessions per week for 4 weeks and daily at-home training. The last 2 weeks consisted solely of at-home practice with telephone support once per week or as needed. The primary mode of intervention was computerized cognitive training using two online cognitive remediation programs (Happy Neuron [sbtpro.com] and BrainHQ [brainhq.com]) that have been widely used in previous studies on cognitive remediation. For each intervention, four exercises from each software program were used, with the only difference being the cognitive domain that was targeted. The interventions were delivered by one therapist in groups with a maximum size of five members, and participants completed the computerized exercises on 9.6-inch Samsung Galaxy tablets. Sessions were divided into practice of computerized exercises (75%) and strategy monitoring through group discussion and completion of a strategy worksheet (25%). Details of the interventions and the computerized exercises are provided in the online supplement.
Randomization and Masking
Participants were randomly assigned (1:1) to treatment conditions with permuted block sizes of four, stratified by recruitment site. Participants were blind to treatment condition and were not provided with details related to the different treatment conditions. Assessors were similarly blind to treatment condition, study hypotheses, and the details of the interventions. There were no breaks in the blind during the study. Data were entered and double-scored by research assistants blind to treatment allocation and study hypotheses.
All outcome measures were assessed at baseline, within 1 week after treatment, and again at follow-up 12 weeks after the end of active treatment. With the exception of case manager-rated community functioning, all assessments were conducted by trained research assistants with a minimum of 1 year’s experience conducting psychological assessments. Symptom assessments were conducted by trained Ph.D.-level graduate students supervised by a licensed clinical psychologist.
Primary Outcome
Community functioning.
The primary outcome measure was real-world functional behavior rated on the Specific Levels of Functioning Scale (
21). The scale was rated by the participant’s case manager or psychiatrist, who was blind to treatment allocation and was not involved in any other assessment procedures. Ratings were made on a 5-point Likert scale according to the frequency of the behavior and the amount of assistance required to perform the behavior. The three domains of interpersonal relationships, daily activities, and work skills were averaged to form a total community functioning score that was transformed to a percentage of the maximum total score, with excellent internal consistency (Cronbach’s alpha=0.91). Higher scores indicate better functioning.
Secondary Outcomes
Functional competence.
Functional competence refers to skills that are important for independent community functioning and was assessed with performance-based simulations of daily tasks. The Canadian Objective Assessment of Life Skills–Brief (
22) assesses three domains of functional activities: time management, domestic activities, and trip planning. Each domain is further subdivided into a procedural knowledge routines subscale, which consists of more basic procedural skills, and an executive operations subscale assessing skills that require higher-level reasoning abilities. Total scores (Cronbach’s alpha=0.78), procedural knowledge routine scores (Cronbach’s alpha=0.70), and executive operation scores (Cronbach’s alpha=0.68) were obtained by summing the respective scores across functional domains and were then converted to age-corrected z-scores based on normative data (
22). Higher scores indicate better functional competence.
Neurocognition.
Neurocognitive performance was assessed with the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (
23), with two modifications. The Mayer-Salovey-Caruso Emotional Intelligence Test, assessing social cognition, was omitted because of the emphasis on neurocognition in this study’s interventions. To increase the number of executive functioning tasks, the Towers Test from the Delis-Kaplan Executive Function System (
24) was substituted for the mazes task to assess problem solving, and the Trail-Making Test, Part B (
25), was included to assess set-shifting ability. Alternate forms were used for measures of verbal and spatial learning and memory because of the susceptibility of these tasks to practice effects. Age-corrected, gender-corrected, and education-corrected z-scores were calculated for domains of processing speed, attention, learning and memory, and working memory as outlined in the manual. An additional domain of executive functioning was calculated from the Towers Test total achievement score and the ratio of Trail-Making Test, Part B, to Trail-Making Test, Part A, performance (
26). A total neurocognitive composite score was then calculated as the mean of the standardized domain scores (Cronbach’s alpha=0.83). Higher scores indicate better neurocognition.
EEG.
EEG was recorded with the Emotiv EPOC system. A detailed description of the EEG recording and analytical methodology is provided in the online supplement. EEG was recorded during eyes-open and eyes-closed resting states, an n-back working memory task, a P300 attention and vigilance task, and a mismatch negativity perceptual task. Theta power was calculated for a 1-back and 2-back task as the difference between theta power during the task and resting state across frontal electrode sites. Theta power was averaged across the two tasks; higher values indicate greater theta power. P300 was calculated as the difference between the mean amplitude in response to target and nontarget stimuli; higher values indicate a greater P300 response. Mismatch negativity was calculated as the difference between the mean amplitude in response to deviant and standard stimuli; more negative values indicate greater mismatch negativity. Detailed descriptions of the EEG tasks are provided in the online supplement.
Supplementary Measures
Clinical symptoms.
The Brief Psychiatric Rating Scale (BPRS) (
27) was used to assess clinical symptoms. The BPRS consists of 18 items rated on a 7-point Likert scale assessing the frequency and severity of symptoms. Factor analyses have produced a consistent five-factor solution assessing domains of affective, positive, negative, resistance, and activation symptoms (
28). All raters were trained through rating interview role playing exercises and videos and were trained to gold-standard ratings by a clinical psychologist (intraclass correlation coefficient=0.98) with ongoing fidelity meetings. The mean item score for each symptom domain is reported, with higher scores indicating more severe psychopathology.
Quality of life.
Self-reported quality of life was measured with the Sheehan Disability Scale (
29), which assesses work and school, social life, and family life and home responsibilities on a 10-point Likert scale. The total score was derived as the mean domain score converted to a percentage of the maximum score (Cronbach’s alpha=0.78). Higher scores indicate poorer quality of life.
Employment.
Current and highest achieved employment status were scored with the Hollingshead Occupational Scale (
30). Lower scores are associated with higher-level employment requiring greater responsibility.
Self-report measures.
The Need for Cognition Scale (
31) assesses the degree to which participants seek cognitively challenging activities in their daily lives (Cronbach’s alpha=0.80); higher scores indicate greater need for cognition. The Generalized Self-Efficacy Scale (
32) assesses general self-efficacy to complete a variety of daily tasks (Cronbach’s alpha=0.87); higher scores indicate greater self-efficacy. The Cognitive Failures Questionnaire (
33) assesses self-identified instances of cognitive difficulties in daily life (Cronbach’s alpha=0.94); higher scores indicate more instances of cognitive difficulties in daily life. Additionally, the Intrinsic Motivation Inventory (
34) (Cronbach’s alpha=0.88) and the Perceived Competence Scale (
35) (Cronbach’s alpha=0.94) were administered after the first treatment session and at the posttreatment assessment to assess intrinsic motivation and perceived competence on the computerized training exercises.
Statistical Analysis
Independent-samples t tests were used to examine differences between groups on baseline demographic and illness variables. Independent-samples t tests were also used to examine differences between individuals who completed the intervention and individuals who discontinued treatment. Primary outcome analyses were intent-to-treat analyses using linear mixed models to examine changes in outcome measures over time by treatment group. Different covariance structures were examined to determine which best fit the data, and the compound symmetry structure was the best fit for all outcome variables. Within-subject Cohen’s d was calculated using the estimated marginal means from the linear mixed models for each group independently based on the change between baseline and each posttreatment assessment.
Results
Participants
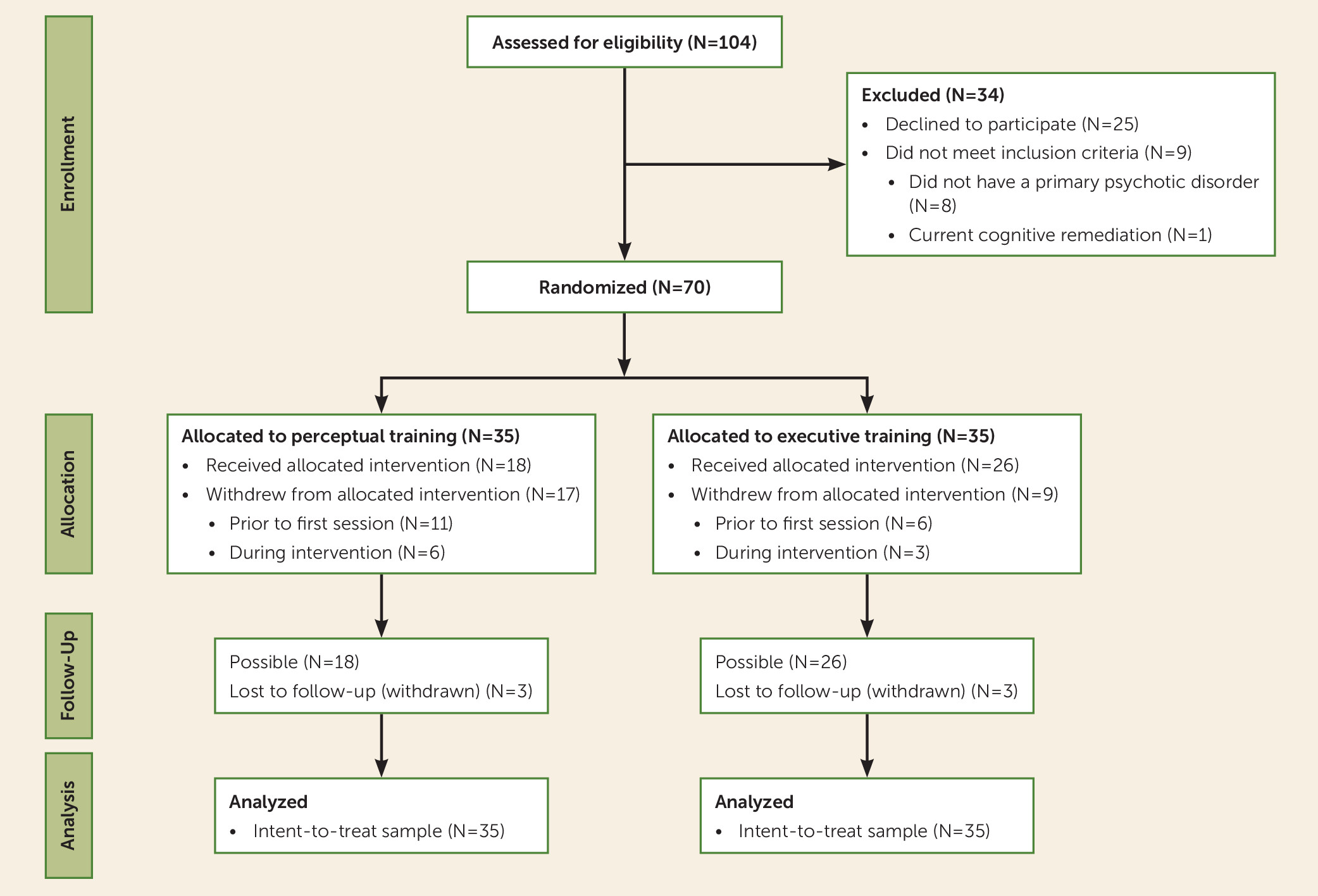
Participant randomization and flow are illustrated in
Figure 1. Comparisons of baseline demographic and illness variables are presented in
Table 1. There were no significant baseline differences between groups. Medication status by group is presented in Table S1 in the
online supplement. Raw data from the neurocognitive and functional competence tests at baseline are presented in Table S2 in the
online supplement. Two participants in each group had previously received cognitive remediation an average of 39.5 months prior to enrollment in this study.
Acceptability
Twenty-six individuals (37%) withdrew from the study before completing treatment. The majority of those who withdrew (65%) did so before attending any treatment sessions. Comparisons between individuals who completed treatment and those who discontinued treatment are presented in Table S3 in the online supplement. Individuals who discontinued treatment tended to be younger, to be currently employed at higher occupation levels, and to have been more recently hospitalized for psychiatric reasons. Participants were also more likely to discontinue treatment if they were assigned to perceptual training than if they were assigned to executive training, with twice as many participants withdrawing from perceptual training (N=6) compared with executive training (N=3) during the intervention window (odds ratio=2.88). Among individuals who completed the interventions, there were no significant differences between groups on number of sessions attended (mean=6.63, SD=1.22) or minutes of homework completed (mean=315.5, SD=334.2).
Intent-to-Treat Analyses
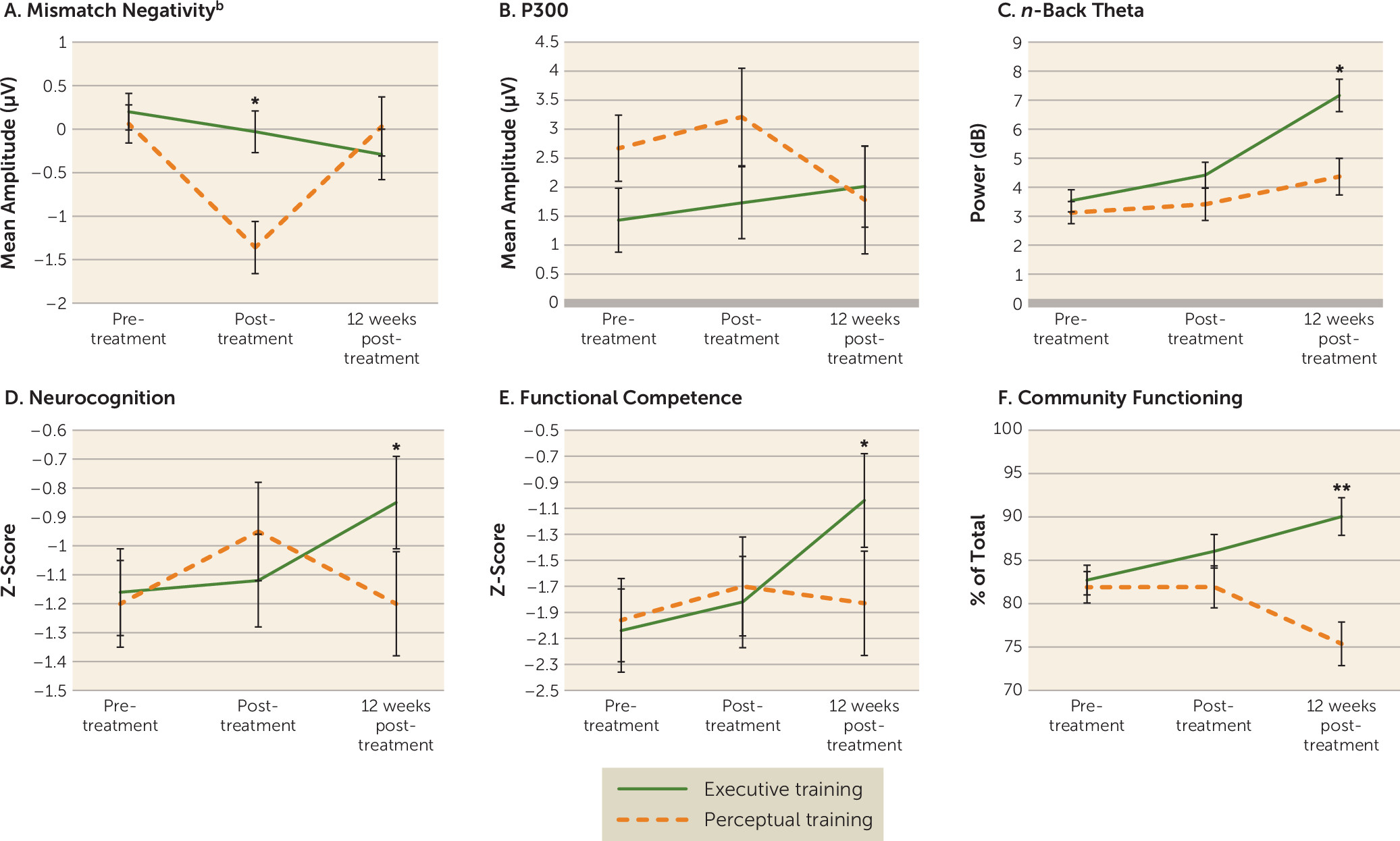
Results from the intent-to-treat analyses are presented in
Table 2 and displayed graphically in
Figure 2. Effect sizes are presented in
Table 3.
Neurophysiology
From baseline to the posttreatment assessment, there were significant main effects of time (F=11.03, df=1, 54.3, p=0.002) and group (F=8.60, df=1, 54.5, p=0.005), such that mismatch negativity amplitude was larger after treatment than at baseline and larger for individuals in perceptual training compared with those in executive training. The interaction between time and group was significant (F=5.69, df=1, 54.3, p=0.021), with the perceptual training group significantly improving from baseline to the posttreatment assessment (F=10.72, df=1, 22.8, p=0.003), but there was no significant improvement in the executive training group. When the baseline and the 12-week follow-up assessment were compared, there were no significant main effects or interactions. Activation by electrode site is presented in Figures S1 and S2 in the online supplement. For the P300 task, there were no significant main effects or interaction at either the posttreatment or the 12-week follow-up assessment compared with baseline. Activation by electrode site is presented in Figures S3 and S4 in the online supplement.
There were no significant main effects or interactions for EEG theta power from baseline to the posttreatment assessment. At the 12-week follow-up, there were significant main effects of time (F=26.16, df=1, 49.4, p<0.001) and group (F=9.65, df=1, 62.3, p=0.003) and a significant time-by-group interaction (F=6.05, df=1, 49.4, p=0.017). Individuals who received executive training had significantly greater theta power at the follow-up compared with baseline (F=38.82, df=1, 24.1, p<0.001), whereas there was no significant change in the perceptual training group (F=2.68, df=1, 25.5, p=0.114).
Neurocognition
For the neurocognitive composite score, there were no significant main effects or interaction from baseline to the posttreatment assessment. At the 12-week follow-up, there was a significant main effect of time (F=4.53, df=1, 39.0, p=0.040) and a significant interaction between treatment condition and time (F=5.67, df=1, 39.0, p=0.022). Individuals in the executive training group improved significantly from baseline (F=28.77, df=1, 22.5, p<0.001), whereas the difference compared with baseline for the perceptual training group was not significant (F=0.003, df=1, 16.4, p=0.957).
Functional Competence
There were no significant main effects or interaction effects on the Canadian Objective Assessment of Life Skills total score or domain score between baseline and the posttreatment assessment. At the 12-week follow-up, there was a significant main effect of time (F=6.51, df=1, 40.0, p=0.015) and a significant interaction between time and treatment condition (F=4.58, df=1, 40.0, p=0.038) on total score. Individuals in the executive training group significantly improved on the functional competence total score from baseline to follow-up (F=14.31, df=1, 22.3, p=0.001), whereas individuals in the perceptual training group did not (F=0.067, df=1, 17.3, p=0.799). The improvement in the executive training group on total functional competence appears to have been driven by a greater improvement on the executive operations subscale compared with the perceptual training group (F=6.21, df=1, 40.2, p=0.017). There was no significant interaction between group and time on the procedural knowledge routines subscale (F=2.01, df=1, 46.4, p=0.162).
Real-World Functioning
There were no significant main effects or interaction on the Specific Levels of Functioning Scale from baseline to the posttreatment assessment. At the 12-week follow-up, there was a significant main effect of treatment condition (F=11.64, df=1, 63.8, p=0.001) and a significant interaction between treatment condition and time (F=12.96, df=1, 46.4, p=0.001). The executive training group significantly improved from baseline to follow-up (F=8.98, df=1, 28.1, p=0.006), whereas the perceptual training group had significantly poorer functioning at follow-up compared with at baseline (F=5.32, df=1, 17.9, p=0.033).
Additional Outcome Measures
There were no significant time-by-treatment interactions for psychiatric symptoms, self-reported quality of life, self-efficacy, cognitive failures, or need for cognition. Results are presented in Table S4 in the online supplement. From baseline to the posttreatment assessment, there was a significant main effect of time for the BPRS affective symptoms subscale (F=6.79, df=1, 43.5, p=0.012) and the negative symptoms subscale (F=0.64, df=1, 49.9, p=0.020), such that both the executive and personal training groups improved. From baseline to the 12-week follow-up, there was a significant main effect of time on the Sheehan Disability Scale (F=5.63, df=1, 45.3, p=0.022) and on the BPRS affective symptoms subscale (F=9.38, df=1, 40.6, p=0.004), the positive symptoms subscale (F=7.61, df=1, 34.6, p=0.009), and the negative symptoms subscale (F=7.57, df=1, 45.3, p=0.008), such that both groups improved.
Discussion
This study is the first, to our knowledge, to directly compare executive and perceptual approaches to training cognition in schizophrenia spectrum disorders, and it includes the broadest assessment battery used to date to examine cognitive remediation spanning domains of neurophysiology, neurocognition, functional competence, and real-world community functioning. In most measures, executive training and perceptual training demonstrated similar improvements immediately after treatment. However, executive training appeared to prime individuals for further improvement after the end of treatment, whereas improvements from perceptual training waned in the months after treatment ended.
The effects of perceptual training compared with executive training appeared most strongly in the mismatch negativity response immediately after the end of active treatment. However, these improvements did not persist 12 weeks later. Conversely, the effects of executive training did not emerge until 12 weeks after the end of treatment, when improvements in neurophysiology, neurocognition, functional competence, and community functioning were observed. Additionally, the magnitude of functional change was comparable to that observed in longer cognitive remediation programs (
36), suggesting that improving higher-level cognitive operations is a critical factor in improving community functioning.
The sleeper effects observed in executive training are consistent with findings in other studies demonstrating continued improvements after the end of treatment (
36). Understanding the mechanisms through which continued improvement occurs, and for whom, will be important areas for further investigation, and capitalizing on these improvements after treatment ends may improve the long-term efficacy of cognitive remediation.
It is possible that the executive training effects were strongest 12 weeks after the end of treatment because the skills and strategies developed during executive training were more functionally relevant and promoted greater engagement in community activities. Previous studies have demonstrated the importance of executive functioning for employment and activities of daily living (
37) in schizophrenia, and cognitively enriched environments are associated with increased neuroplasticity (
38). Thus, executive training may initially facilitate greater engagement with more cognitively stimulating environments as a result of the greater functional relevance of training, and then, through engagement with a more stimulating environment, continued cognitive and functional improvement may occur. Meta-analytic evidence has suggested that cognitive remediation produces the greatest improvements when delivered adjunctively to other psychiatric rehabilitation (
5,
6), promoting a cognitively stimulating environment, but further studies are needed to confirm this interpretation. Development of structured methods for recording the cognitive stimulation associated with additional therapeutic or everyday behaviors is needed to help address this issue.
There are potential clinical and health care policy implications of our findings. In a disorder characterized by severe and persistent functional disability, a brief course of executive training improved neurophysiological, cognitive, and functional abilities compared with another widely used form of cognitive remediation. Not only was executive training more effective than perceptual training, it was also delivered in a smaller dose than the majority of cognitive remediation programs described in the literature, which can last as long as 2 years (
17). Eight sessions of executive training supplemented with at-home training should be feasible within most health care systems, and our results suggest that a brief executive training intervention may stimulate ongoing cognitive and functional improvements. The potential economic implications of such an intervention are vast, although a larger trial is needed to examine this issue.
Our findings should be considered in light of limitations and considerations. The protocolized treatment dose (28 hours) is not inconsistent with that of many cognitive remediation studies. However, the time frame of 6 weeks is shorter than most. Any interaction of the strategy monitoring procedure with the specific type of cognitive training is unclear, and future mechanistic studies could examine this. There was no control condition; however, the observed effects were larger than the typically observed practice effects on measures such as the MATRICS Consensus Cognitive Battery (SD=0.2) (
39). The large attrition rate is consistent with many interventions for severe mental illness and speaks to the challenges of disseminating interventions from research settings to clinical practice. Greater attrition was observed in the perceptual training group than in the executive training group, perhaps because the executive training exercises were more enjoyable and motivating. However, intent-to-treat analyses were used to control for this. When cognitive remediation has included personalized goal setting and practical approaches to linking cognitive gains to everyday functions, retention has been shown to be higher (
40). The majority of participants in the present study were Caucasian and had mild symptoms, making it difficult to determine whether treatment effects would generalize more broadly. We were also limited in the EEG markers that we could examine with the Emotiv EEG system. The Emotiv system allowed for flexibility in assessing participants in community settings but is unable to detect early perceptual components, such as the P50, which have been examined in previous studies (
12).
Conclusions
Although perceptual training may produce short-term improvements in neurophysiology and neurocognition during active treatment, treatment effects from executive training techniques are more likely to transfer to community functioning and persist. In fact, executive training appears to prime the individual for further functional improvement once treatment ends, suggesting that a brief dose of executive training may provide ongoing benefits and should be a prioritized treatment target within cognitive remediation.
Acknowledgments
The authors thank Amanda Shamblaw, Irene Hong, Abi Muere, Mashal Haque, Pauline Leung, Laura Lambe, Robyn Jackowich, Stephanie Gauvin, Tanya Tran, Kenzie Bender, Lilian Laferriere, Mandy Hagen, and Jessie Eriksen for their assistance conducting assessments; Ellie Lambert, Adriana Farcas, Catherine Karremans, Sue Streight, Paddy Dolphin, Kathleen Hunn, Mary Anne Hensman, Jane Keates, and Jessica Pang for referring participants to the study; and Talia Leibovitz, Lauren Harper, Caroline Uchida, Kaylynn Brant, Lindsay Simourd, Kyra McGovern, Mara Dempsey, Minha Haque, Natasha Barich, Nicole Sanvido, Samntha Irwin, and Tarindi Welikala for assistance with data scoring and entry.