When Discontinuing SSRI Antidepressants Is a Challenge: Management Tips
What are Discontinuation Symptoms?
How Common are Discontinuation Symptoms?
| Drug and Type of Report | Study Findings |
|---|---|
| Paroxetine | |
| Clinical trials | Rosenbaum et al. (52): After paroxetine discontinuation for up to 8 days, 66% of patients (N=82) developed significant discontinuation symptoms. The mean severity of discontinuation symptoms was highest for paroxetine, intermediate for sertraline, and lowest for fluoxetine. |
| Baldwin et al. (55): A higher overall severity of discontinuation symptoms was observed with paroxetine compared with escitalopram (study N=323). Over 1 week immediately after discontinuation, specific symptoms were reported more often with paroxetine than with escitalopram: feeling tense (paroxetine=24.5%, escitalopram=12.7%), confusion (paroxetine=22.7%, escitalopram=10.4%), nausea (paroxetine=15.5%, escitalopram=5.2%), forgetfulness (paroxetine=15.5%, escitalopram=4.5%), sweating more than usual (paroxetine=13.6%, escitalopram=3.7%), shaking (paroxetine=11.8%, escitalopram=3.0%), sudden panic (paroxetine=8.2%, escitalopram=1.5%), and diarrhea (paroxetine=9.1%, escitalopram=0.7%). | |
| Michelson et al. (53): Interruption of paroxetine for 5 days was associated with worsening of 13 of 17 items on a solicited adverse event rating scale (N=36), with dizziness being the most common (reported by 57.1%). | |
| Judge et al. (56): Discontinuation of paroxetine for 3–5 days was associated with significantly higher severity of new-onset or worsened symptoms and worsening of depressive symptoms and functional impairments than discontinuation of fluoxetine (study N=150). Dreaming (36%) and dizziness (34%) were the most commonly reported discontinuation symptoms. | |
| Bogetto et al. (57): Discontinuation symptoms were present in 22 of 52 patients (42.3%) who discontinued paroxetine. | |
| Fava et al. (61): Even after gradual discontinuation, five of nine patients on paroxetine developed discontinuation syndrome, which persisted for more than 1 month in three of the patients. | |
| Montgomery et al. (50): Discontinuation of paroxetine was associated with more new symptoms (mean=7.3) in 1 week than continuation of paroxetine (mean=3.5) (study N=192). | |
| Oehrberg et al. (51): After 12 weeks of double-blind paroxetine and placebo treatment, 19 of 55 patients (34.5%) on paroxetine and seven of 52 (13.5%) reported new symptoms during a 2-week blinded discontinuation with placebo. | |
| Case reports | Barr et al. (22): Three of six patients developed discontinuation symptoms 3–7 days after paroxetine discontinuation despite a 1- to 2-week taper. |
| Keuthen et al. (23): Five of 13 patients developed discontinuation symptoms during paroxetine taper or 2–14 days after last dose. These symptoms lasted from 4 days to 2 weeks. In one patient, reinitiation of paroxetine abruptly terminated discontinuation symptoms. | |
| Pyke (27): Discontinuation symptoms developed in a patient 3 days after discontinuation of paroxetine (10–20 mg/day); symptoms resolved in 3 weeks. | |
| Phillips (28): Discontinuation symptoms developed in a patient 3 days after discontinuation of paroxetine (20 mg/day, prescribed for 5 weeks) and resolved on reinitiation of paroxetine but not with initiation of sertraline. | |
| DeBattista et al. (29): In two patients, discontinuation symptoms developed 1–2 days after discontinuation of paroxetine (10 mg/day) and resolved after paroxetine reinitiation. | |
| Frost and Lal (32): Two days after discontinuation of paroxetine (10–20 mg/day), two patients reported electric shock–like sensations, which resolved over 3 weeks. | |
| Dominguez and Goodnick (33): Discontinuation symptoms developed in three patients after discontinuation of paroxetine (40 mg/day). | |
| Bloch et al. (38): Hypomania developed in a patient after abrupt discontinuation of paroxetine (50 mg/day). | |
| Coupland et al. (58): In a retrospective review, 10 of 50 patients who discontinued paroxetine developed discontinuation symptoms. | |
| Peeters et al. (34): Severe discontinuation symptoms, including fever, developed in a patient on gradual reduction and discontinuation of paroxetine, which improved with medication reinitiation. | |
| Bryois et al. 1994: Discontinuation symptoms occurred in a patient after discontinuation of paroxetine. | |
| Fagan (36): Shock-like symptoms occurred in two patients on discontinuation of paroxetine. | |
| Fava and Grandi (24): Discontinuation symptoms started in three patients 7–10 days after discontinuation of paroxetine and persisted for 7–10 days. | |
| Sertraline | |
| Clinical trials | Rosenbaum et al. (52): After sertraline discontinuation for up to 8 days, 60% of patients (N=79) developed significant discontinuation symptoms. |
| Michelson et al. (53): Interruption of sertraline for 5 days was associated with worsening of three of 17 items on a solicited adverse event rating scale (N=34), with dizziness (reported by 42.4%) being the most common. | |
| Case reports | Louie et al. (26): Abrupt discontinuation of sertraline (100 mg/day, prescribed for 5 weeks) led to discontinuation symptoms after 2 days, which resolved with reinitiation of sertraline at 25 mg/day. These symptoms recurred to a milder degree after each dose reduction during a 12-week taper. |
| Coupland et al. (58): In a retrospective review, one of 45 patients who discontinued sertraline developed discontinuation symptoms. | |
| Frost and Lal (32): Sensation of electric shocks was reported by a patient 1 day after discontinuation of sertraline (50 mg/day, which had been gradually tapered down from 150 mg/day over 8 weeks) and persisted with lessening intensity for 13 weeks. | |
| Leiter et al. (37): Discontinuation symptoms started in two patients 1–2 days after discontinuation of sertraline (100–150 mg/day) and lasted up to 1 month. | |
| Fava and Grandi (24): Discontinuation symptoms started in a patient 5 days after discontinuation of sertraline (50 mg/day) and persisted for 1 week. | |
| Citalopram | |
| Clinical trials | Fava et al. (61): One of three patients (33.3%) had four or more discontinuation symptoms after stopping citalopram; symptoms resolved within 1 month. |
| Case reports | Young et al. (54): Two Committee on the Safety of Medicines reports of new-onset or worsened symptoms after discontinuation. |
| Escitalopram | |
| Clinical trials | Baldwin et al. (55): Escitalopram was better tolerated and was associated with fewer study dropouts than paroxetine. Rates of patients reporting individual discontinuation symptoms have been presented earlier with paroxetine. |
| Case reports | De Berardis et al. (39): Manic symptoms emerged after discontinuation of low-dose escitalopram in a patient with major depression; symptoms resolved on reinitiation. |
| Fluoxetine | |
| Clinical trials | Rosenbaum et al. (52): After fluoxetine discontinuation for up to 8 days, 14% of patients (N=81) developed significant withdrawal symptoms. |
| Michelson et al. (53): Interruption of fluoxetine for 5 days was associated with no worsening of any symptoms on a 17-item solicited adverse event rating scale (N=37). | |
| Judge et al. (56): Discontinuation of fluoxetine for 3–5 days was better tolerated than discontinuation of paroxetine. Fatigue (17%) and nervousness (16%) were the most commonly reported new-onset or worsened symptoms. | |
| Bogetto et al. (57): Discontinuation symptoms were present in four of 45 patients (8.9%) who discontinued fluoxetine. | |
| Fava et al. (61): One of three (33.3%) patients had four or more discontinuation symptoms after stopping fluoxetine; symptoms resolved within 1 month. | |
| Zajecka et al. (60): Of 395 fluoxetine responders, 96 were abruptly switched to placebo; those switched to placebo had rates of new or worsened adverse events similar to those who continued on fluoxetine (N=299). Discontinuation of fluoxetine was associated with a slightly higher rate of dizziness at 6 weeks (5% compared with 1%). | |
| Case reports | Stoukides and Stoukides (21): Discontinuation symptoms occurred in a patient 1–2 days after sudden discontinuation of a 6-month course of fluoxetine; symptoms responded to symptomatic management. |
| Einbinder (30): Discontinuation symptoms developed in a patient 3 days after discontinuation of fluoxetine (40 mg/day) on two separate occasions and resolved with reinitiation of fluoxetine each time. Discontinuation symptoms recurred on discontinuation of fluoxetine (5 mg/day) after a taper period. However, using liquid fluoxetine, no discontinuation symptoms occurred on discontinuation of fluoxetine at a dosage of 2.5 mg/day. | |
| Coupland et al. (58): In a retrospective review, none of 20 patients who discontinued fluoxetine developed discontinuation symptoms. | |
| Berlin et al. (31): Discontinuation symptoms occurred in three patients 2–25 days after discontinuation of fluoxetine (20–40 mg/day) and lasted 4–8 weeks. | |
| Blum et al. (62): Delirium developed in a patient after discontinuation of fluoxetine and resolved immediately on reinitiation. | |
| Fan and Liu (63): Delirium developed in a patient 2 days after discontinuation of fluoxetine (20 mg/day) and gradually improved on reinitiation of fluoxetine. |
How Do Discontinuation Symptoms Present?
| System | Signs and Symptoms |
|---|---|
| General | Chills, malaise, flu-like symptoms, fatigue, lethargy, fever, diaphoresis |
| Eyes | Blurred vision, eye movement abnormalities, sore eyes, eye twitch |
| Ears, nose, mouth, and throat | Tinnitus, rhinorrhea, sinus congestion, nasal congestion, increased salivation |
| Respiratory | Shortness of breath |
| Cardiovascular | Palpitation, tachycardia, elevation in systolic and diastolic blood pressure |
| Gastrointestinal | Nausea, vomiting, diarrhea, abdominal pain, stomach cramp, abdominal bloating |
| Genitourinary | Genital hypersensitivity, premature ejaculation |
| Musculoskeletal | Sore muscles, myalgia, arthralgia, muscle cramps |
| Skin and hair | Pruritus |
| Neurological | Disequilibrium (vertigo, dizziness, light-headedness, gait instability, and ataxia), sensory disturbances (unusual sensitivity to sound, electric shock–like sensations, paresthesia, tinnitus, dysgeusia, and brain zaps), neuromuscular symptoms (acute dystonia, myoclonus, tremor, shaking, akathisia), and cognitive symptoms (delirium, amnesia, memory impairments, disorientation, and confusion) |
| Psychiatric | Worsening of mood (dysphoria, hypomania, depression, bouts of crying, tearfulness, impulsiveness, irritability, agitation, anger attacks, mood swings, impaired concentration, muscle tension, suicidal and homicidal ideations), exacerbation of anxiety (tension, panic, and generalized anxiety), sleep disruption (insomnia, hypersomnia, vivid dreams, nightmares, disrupted circadian rhythm), and perceptual impairments (depersonalization, derealization, hypnogogic hallucinations, unusual visual sensations such as geometric shapes and colors, auditory and visual hallucinations) |
What is the Differential Diagnosis for Discontinuation Symptoms?
What is the Pathophysiology of Discontinuation Symptoms?
When Do Discontinuation Symptoms Occur and How Long Do They Last?
Who is At Risk Of Discontinuation Symptoms?
How are Discontinuation Symptoms Distinct From Relapse or Recurrence?
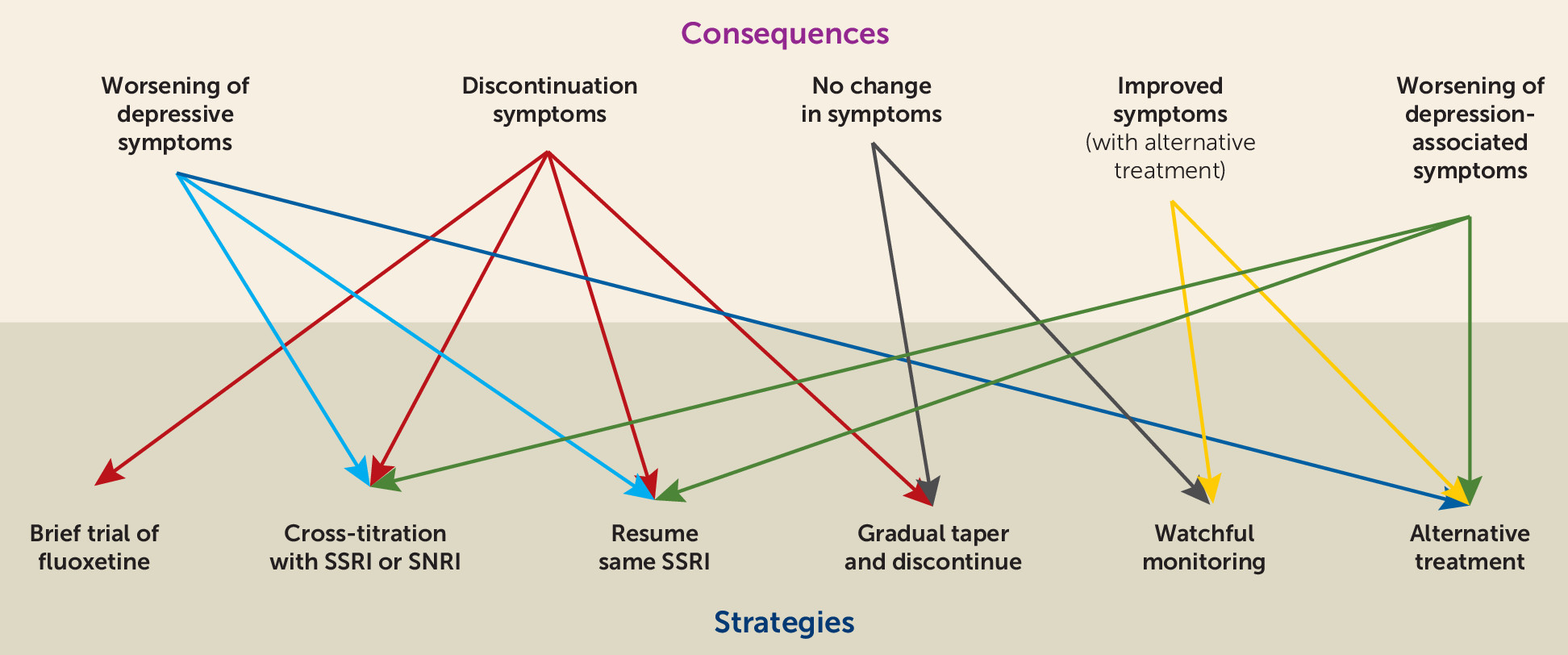
What are the Best Strategies to Manage Discontinuation Symptoms?
Recommendations
Acknowledgments
References
Information & Authors
Information
Published In
History
Keywords
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

