Reducing Suicidal Ideation Through Insomnia Treatment (REST-IT): A Randomized Clinical Trial
Abstract
Objective:
Methods:
Results:
Conclusions:
Methods
Participants
Randomized Assignment and Masking
Clinical Measurements
Secondary Outcome Variables
Sleep metrics.
Depression severity.
Hopelessness.
Health-related quality of life.
Overall status and treatment response.
Antidepressant treatment resistance.
Adverse events.
Actigraphy.
Statistical Analyses
Results
Baseline
| Characteristic | Controlled-Release Zolpidem (N=51) | Placebo (N=52) | Overall (N=103) | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 39.7 | 14.5 | 41.2 | 12.0 | 40.5 | 13.2 |
| Body mass index | 28.3 | 6.4 | 28.2 | 5.6 | 28.2 | 6.0 |
| N | % | N | % | N | % | |
| Female | 32 | 63 | 32 | 62 | 64 | 62 |
| Clinic | ||||||
| 1 | 23 | 45 | 27 | 52 | 50 | 49 |
| 2 | 15 | 29 | 15 | 29 | 30 | 29 |
| 3 | 13 | 25 | 10 | 19 | 23 | 22 |
| Race or ethnicity | ||||||
| Caucasian | 30 | 59 | 33 | 63 | 63 | 61 |
| African American | 12 | 24 | 16 | 31 | 28 | 27 |
| Hispanic | 4 | 8 | 1 | 2 | 5 | 5 |
| Other | 5 | 10 | 2 | 4 | 7 | 7 |
| Antidepressant trials in current episode | ||||||
| Zero | 29 | 57 | 30 | 58 | 59 | 57 |
| One | 14 | 27 | 12 | 23 | 26 | 25 |
| Two or more | 8 | 16 | 10 | 19 | 18 | 17 |
| Lifetime suicide attempt(s) | 15 | 29 | 16 | 31 | 31 | 30 |
| Posttraumatic stress disorder | 13 | 25 | 16 | 31 | 29 | 28 |
| Obsessive-compulsive disorder | 2 | 4 | 3 | 6 | 5 | 5 |
| Generalized anxiety disorder | 20 | 39 | 21 | 41 | 41 | 40 |
| Panic disorder with agoraphobia | 9 | 18 | 8 | 15 | 17 | 17 |
| Panic disorder without agoraphobia | 10 | 20 | 7 | 14 | 17 | 17 |
| Mean | SD | Mean | SD | Mean | SD | |
| Scale for Suicide Ideation: suicide ideation score | 12.2 | 5.3 | 11.8 | 5.3 | 12.0 | 5.3 |
| N | % | N | % | N | % | |
| Scale for Suicide Ideation: suicide attempts | ||||||
| Never | 36 | 71 | 36 | 69 | 72 | 70 |
| Once | 8 | 16 | 8 | 15 | 16 | 16 |
| Two or more times | 7 | 14 | 8 | 15 | 15 | 15 |
| Scale for Suicide Ideation: death wish | ||||||
| No suicide attempt | 36 | 71 | 36 | 69 | 72 | 70 |
| Low | 3 | 6 | 3 | 6 | 6 | 6 |
| Moderate | 6 | 12 | 4 | 8 | 10 | 10 |
| High | 6 | 12 | 9 | 17 | 15 | 15 |
| Mean | SD | Mean | SD | Mean | SD | |
| Columbia–Suicide Severity Rating Scale (C-SSRS) past-week ideation intensity | 1.71 | 1.03 | 1.58 | 1.02 | 1.64 | 1.02 |
| N | % | N | % | N | % | |
| C-SSRS: a lot of difficulty or inability to control thoughts | 6 | 12 | 12 | 23 | 18 | 18 |
| C-SSRS: deterrents probably or definitely stopped suicide attempt | 43 | 86 | 35 | 67 | 78 | 76 |
| Clinical Global Impressions Scale, severity scale | ||||||
| Mildly ill | 3 | 6 | 0 | 0 | 3 | 3 |
| Moderately ill | 33 | 65 | 32 | 62 | 65 | 63 |
| Markedly ill | 13 | 25 | 18 | 35 | 31 | 30 |
| Severely ill | 2 | 4 | 2 | 4 | 4 | 4 |
| Mean | SD | Mean | SD | Mean | SD | |
| Hamilton Depression Rating Scale score | 28.7 | 4.7 | 29.6 | 7.0 | 29.1 | 5.9 |
| Insomnia Severity Index score | 20.7 | 4.0 | 21.0 | 4.3 | 20.9 | 4.1 |
| Beck Hopelessness Scale score | 13.4 | 4.8 | 12.7 | 4.6 | 13.1 | 4.7 |
| Disturbing Dream and Nightmare Severity Index score | 10.5 | 8.8 | 10.2 | 7.7 | 10.3 | 8.2 |
| Dysfunctional Beliefs and Attitudes About Sleep Scale score | 6.7 | 1.6 | 6.7 | 1.5 | 6.7 | 1.6 |
| Behavior and Symptom Identification Scale 32, daily living and role functioning score | 2.4 | 0.6 | 2.2 | 0.7 | 2.3 | 0.6 |
| Behavior and Symptom Identification Scale 32, relationship to self and others score | 2.5 | 0.6 | 2.2 | 0.7 | 2.3 | 0.7 |
| Mini-Mental State Examination score | 29.6 | 0.8 | 29.3 | 1.0 | 29.4 | 0.9 |
| Epworth Sleepiness Scale score | 7.9 | 5.0 | 8.5 | 4.7 | 8.2 | 4.9 |
Randomized Treatment
Outcomes
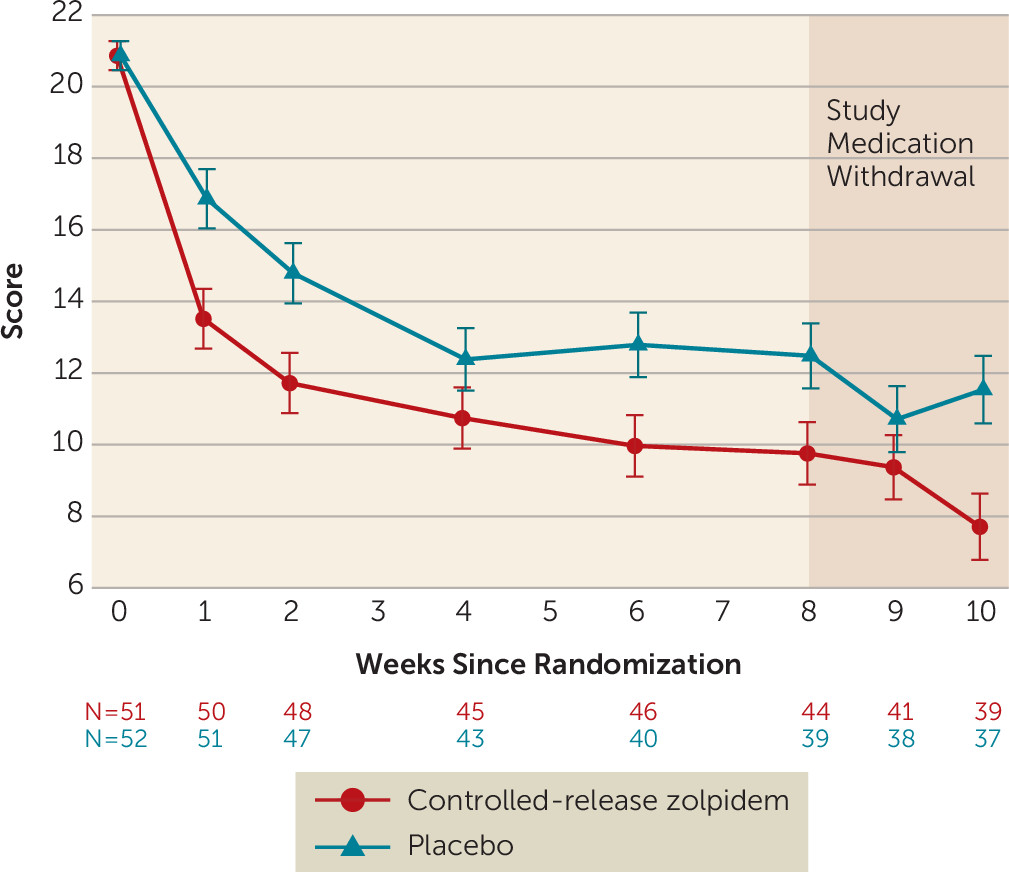
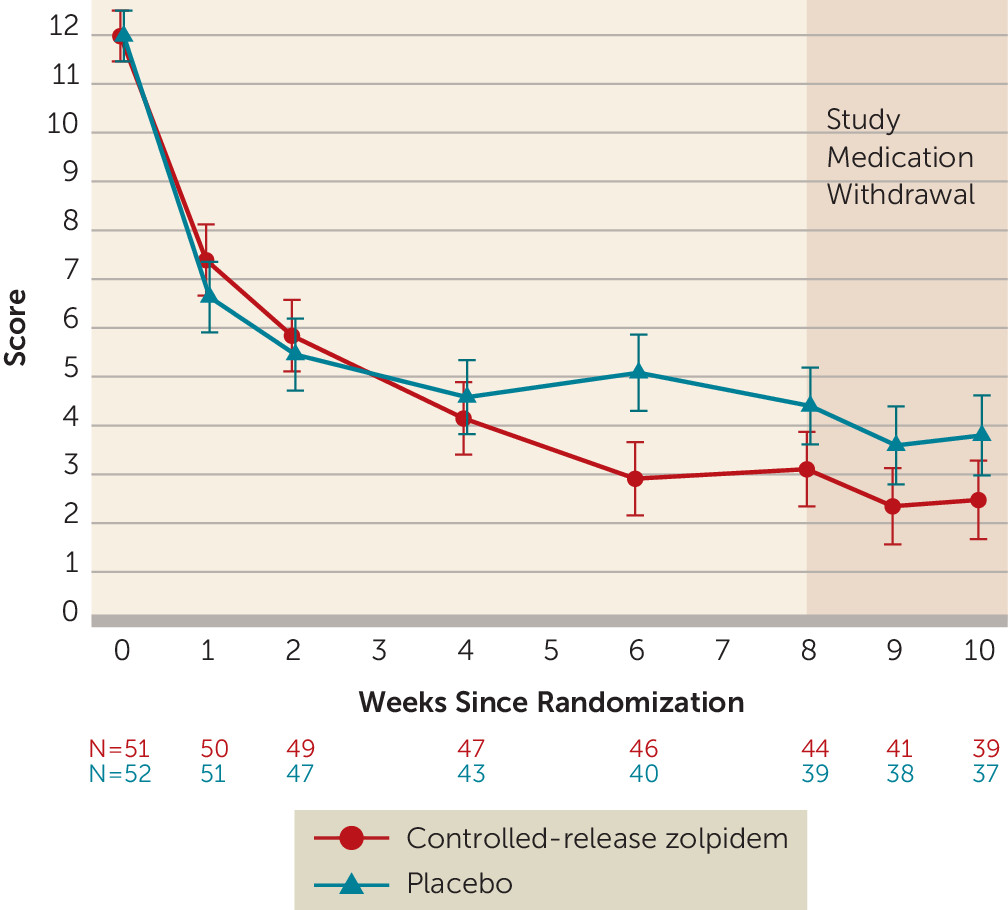
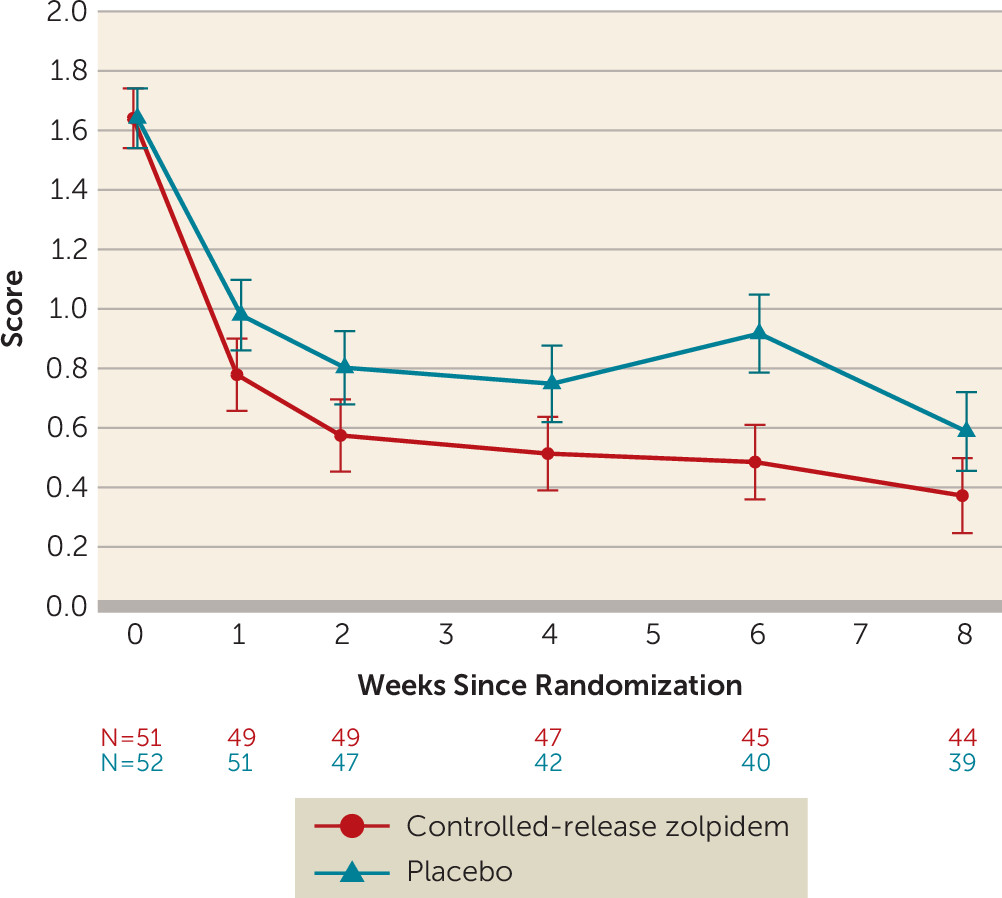
Follow-Up
Adverse Events
Discussion
Conclusions
Acknowledgments
Footnote
Supplementary Material
- View/Download
- 46.15 KB
References
Information & Authors
Information
Published In
History
Keywords
Authors
Competing Interests
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

