Heterogeneity in schizophrenia and other psychotic disorders is a major challenge for understanding the relevant biology and developing new treatments. Differing trajectories of development prior to illness onset are an important dimension of this heterogeneity (
1), reflecting poorly understood genetic and environmental influences (
2,
3) and yet predicting some of the clinical, functional, and biological characteristics of affected adults (
4,
5). Three developmental trajectory subtypes are frequently described: one including those with a lifelong history of impaired social, behavioral, and/or intellectual functioning and an insidious progression toward psychotic illness; a second including individuals with a benign developmental course through adolescence followed by a relatively abrupt onset of psychotic symptoms; and a third including individuals who experience a prominent prodromal phase that begins and progresses during adolescence before transitioning into psychosis. These distinctions have been linked to important course, symptom, and outcome variables (
6,
7).
Cognitive ability has often served as a developmental marker in psychotic disorders. Abundant research demonstrates histories of academic difficulties, attention disorders, and cognitive testing differences in many children and adolescents who will later develop schizophrenia (
8–
10), as well as relatively stable cognitive performance after diagnosis (
11). A strategy for using data from adult patients to identify distinct trajectories of cognitive development takes advantage of well-studied patterns on two estimates of intellectual ability: irregular word reading (e.g., the Wide-Range Achievement Test [WRAT] reading subtest [
12]) and full-scale IQ (e.g., the Wechsler Adult Intelligence Scale [WAIS] batteries [
13]). These measures are generally commensurate in healthy adults (
14). However, many people with schizophrenia diverge from the typical pattern, performing at near normal levels on word reading while showing marked impairment on full-scale IQ. This pattern is thought to reflect the maturation and crystallization of word reading skill in advance of the typical time frame for the onset of acute psychotic symptoms, in contrast with the continuing developmental sensitivity of the skills underlying full-scale IQ, and has prompted the use of word reading performance after diagnosis as a proxy for premorbid IQ (
14,
15).
Variation in developmental history has been a target for data-driven subgrouping methods, such as cluster analysis, which parse heterogeneous psychotic disorder samples into subgroups that may be more biologically and behaviorally distinct and treatment relevant (
6,
16). Weickert et al. (
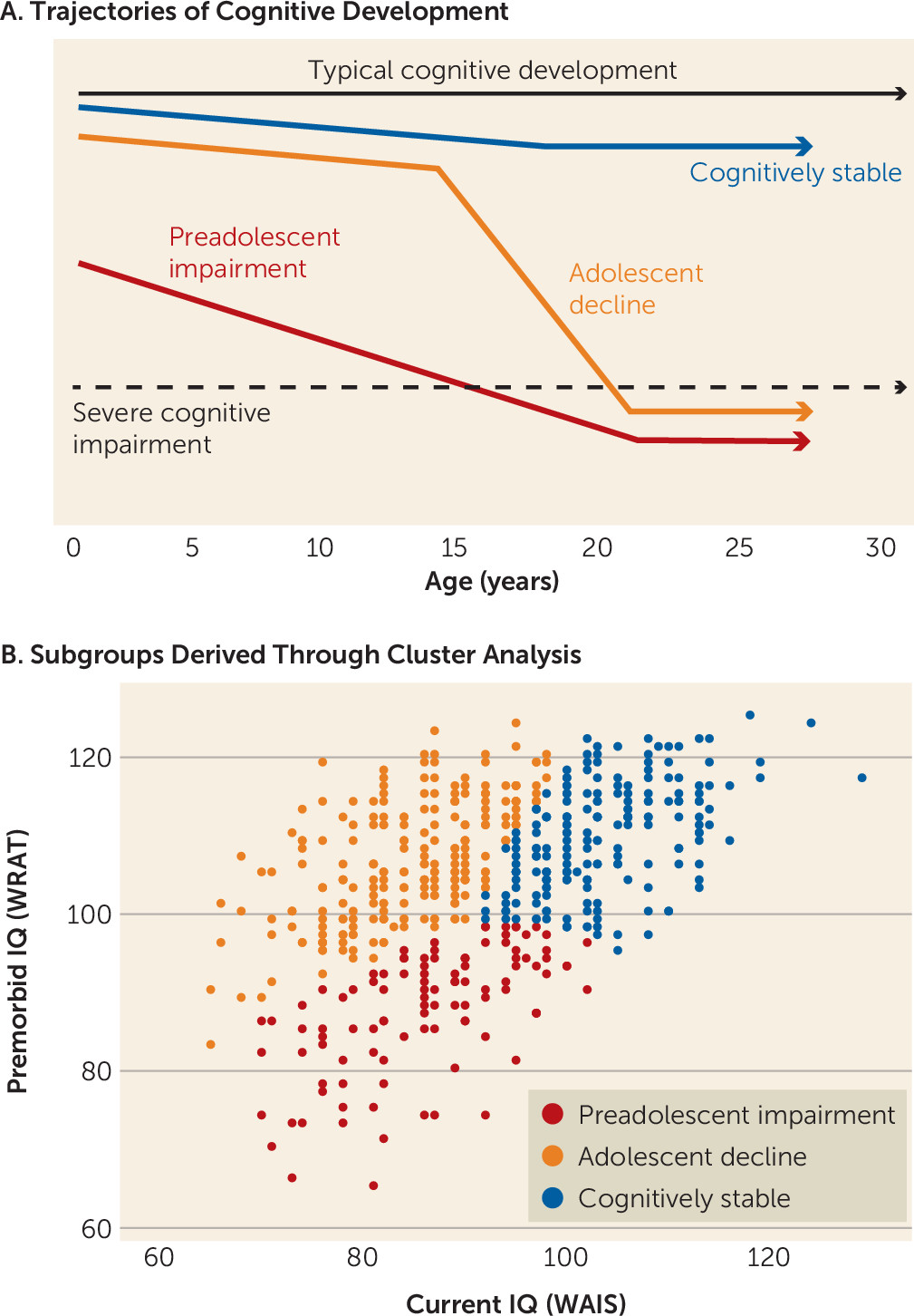
17) used cluster analysis in 117 schizophrenia patients to show that current (WAIS) and premorbid (WRAT) IQ performance patterns distinguished three subgroups: one with low premorbid and current IQ, suggestive of preadolescent cognitive development issues; one with high premorbid and current IQ, indicating a more stable course of cognitive development; and one with high premorbid IQ but low current IQ, highlighting disruptions to cognitive development during adolescence in particular. These subgroups parallel the broad developmental trajectory subtypes described earlier, reflecting distinct trajectories of
cognitive development in schizophrenia (depicted schematically in
Figure 1A). In studies following the Weickert et al. study, the premorbid/current IQ subgrouping strategy has proven to be replicable and has shown associations with clinical course, symptom profiles, and functional outcomes (
18–
21). Regarding biological substrates, recent studies have reported subgroup differences in intracranial and total brain volume (
22,
23). To our knowledge, no studies have examined the association of premorbid/current IQ subgroups with genome-wide polygenic scores (PGSs), which aggregate the effects of thousands of common genetic markers on particular phenotypes (
24,
25).
Our main goals here were to derive and characterize IQ-based subgroups in a large schizophrenia sample, testing the proposition that these subgroups reflect three distinct trajectories of cognitive development, and to show contrasting profiles of genetic influence—represented here by four PGSs—across the subgroups. Different PGSs can be derived simultaneously in a given sample using results from various genome-wide association studies (GWASs), thus allowing the construction of profiles of influence across multiple genetic dimensions (
25). Given that our subgroups were defined by schizophrenia diagnosis and IQ performance patterns, we expected differences in profiles of PGSs for schizophrenia, cognition, educational attainment, and attention deficit hyperactivity disorder (ADHD) that converged with our IQ-based subgrouping. We reasoned that the symptom and outcome differences that have been reported in earlier schizophrenia cognitive subgroup studies (
18–
21) may reflect, in part, differences in underlying schizophrenia genetics. We reasoned further that PGSs for ADHD, cognition, and education would covary in schizophrenia with patterns of current versus premorbid IQ.
Discussion
Our goals in this study were, first, to use an IQ-based strategy in a large and extensively phenotyped schizophrenia sample to identify and characterize subgroups with different pre-diagnosis trajectories of cognitive development and, second, to test whether the profiles of four polygenic scores—separately summarizing the influence of common genetic variants associated with schizophrenia, general cognition, educational attainment, and ADHD—differed by subgroup. The resulting IQ patterns and PGS profiles were consistent with hypotheses and congruent in interesting ways. Cluster analysis based only on “premorbid” (WRAT) and current (WAIS) IQ strongly supported a three-subgroup model, similar to findings in earlier studies (
18,
20,
21), with cognitively stable, adolescent-decline, and preadolescent-impairment subgroups. Distinct cognitive, clinical, and functional characteristics across subgroups helped validate the guiding cognitive development trajectories framework. Finally, profiles of the four PGSs showed a remarkable convergence with the developmental framework and with subgroup characteristics.
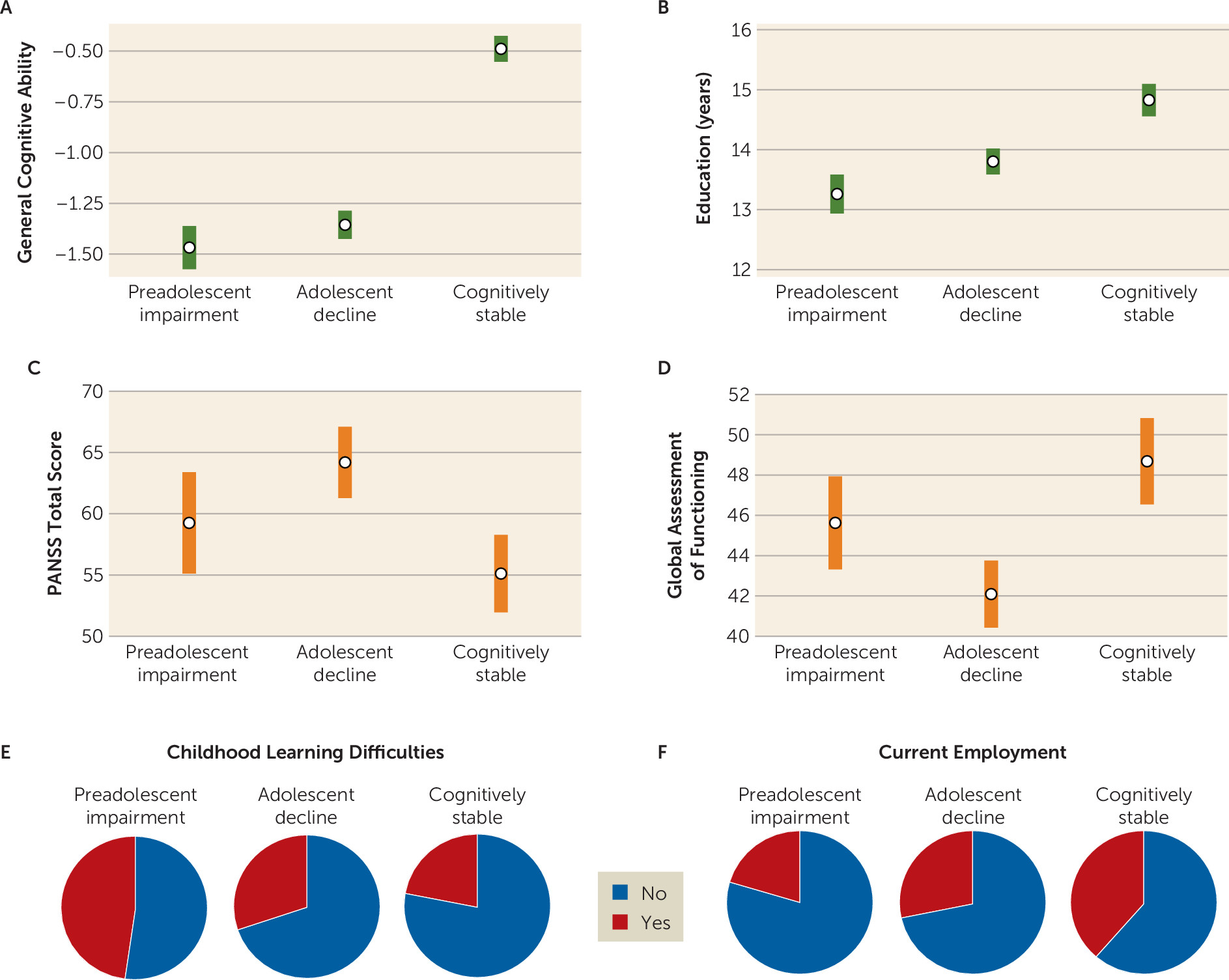
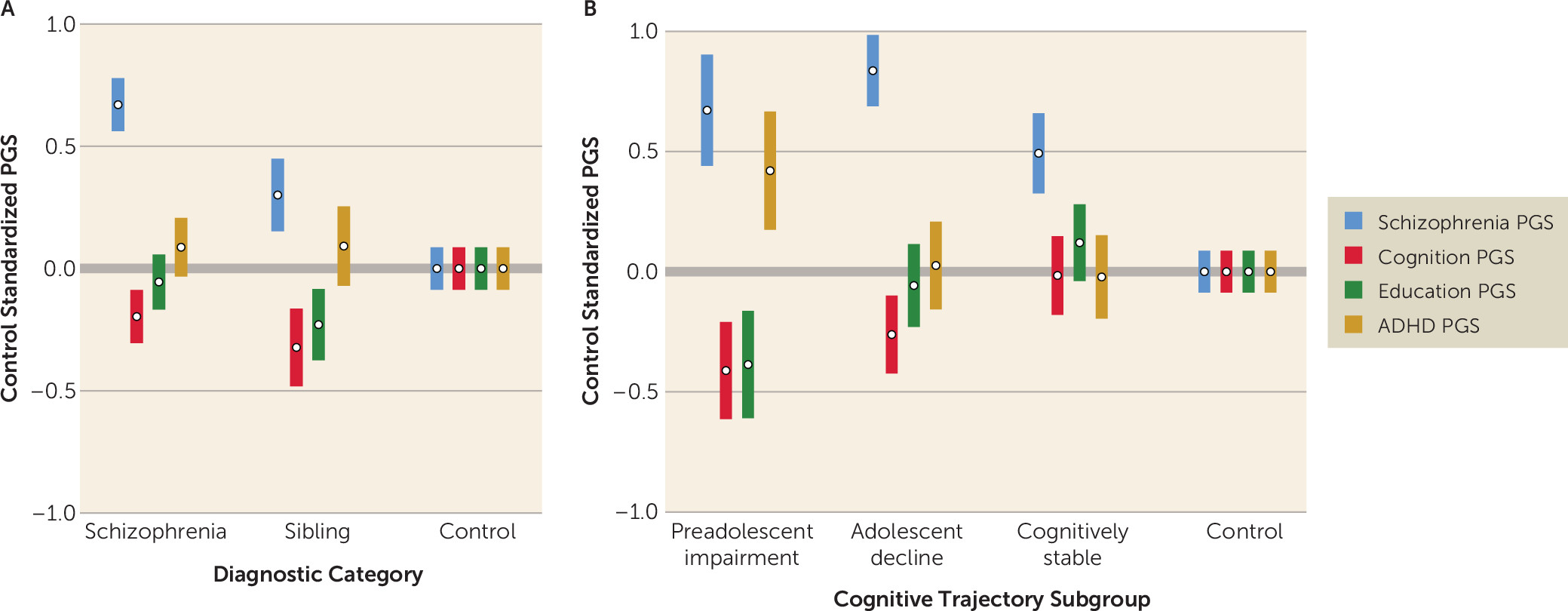
For 37% of the sample, relatively strong performance on both the WRAT and the WAIS suggested a stable cognitive development trajectory, with good early-life cognitive and educational functioning and a more limited impact of emerging psychosis on cognition. Subgroup clinical and functional characteristics in adulthood—beyond WRAT and WAIS results—indicated that individuals in this subgroup had a milder course of illness with relatively lower levels of schizophrenia symptoms (particularly negative symptoms), a strong advantage in general cognitive performance, and higher levels of employment than their peers. The PGS profile for the cognitively stable subgroup was, likewise, relatively more benign than the profiles for the other schizophrenia subgroups. Individuals in this group were similar to control subjects on PGSs for cognition and ADHD. They showed a slight advantage on education PGS, which may relate to a previously reported and counterintuitive positive association between schizophrenia and education PGS (
35,
38). These individuals were only disadvantaged relative to control subjects on schizophrenia PGS.
For the adolescent-decline subgroup, which accounted for 44% of the sample, the matrix of findings was quite different. For these individuals, premorbid IQ in the normal range combined with substantially impaired current IQ suggested a disruption of cognitive development during adolescence, likely overlapping with psychosis prodrome and onset. Illness course and outcome for this subgroup were unfavorable. As adults, in addition to broadly impaired cognitive performance, members of the adolescent-decline subgroup had the most severe schizophrenia symptoms among the subgroups, especially positive symptoms, as well as low levels of employment and the lowest Global Assessment of Functioning ratings. Those in the adolescent-decline group also showed a distinct and unfavorable PGS profile, with a significant disadvantage in terms of schizophrenia and cognition PGSs relative to control subjects and those in the cognitively stable subgroup.
The preadolescent-impairment subgroup, comprising 19% of the sample, was also distinctive. Substantial impairment in both WRAT and WAIS performance in this group suggested early-life divergence from typical cognitive development. Supporting this interpretation, the subgroup had the highest rates of childhood learning problems and the fewest years of education completed. These individuals also had globally impaired cognition and low rates of employment in adulthood. Symptoms were intermediate relative to the cognitively stable and adolescent-decline subgroups. Individuals in the preadolescent-impairment subgroup showed a generalized profile of unfavorable PGSs across the four phenotypes. They were at a significant disadvantage in all PGSs relative to control subjects, and in all but schizophrenia PGS relative to the cognitively stable subgroup. It is particularly striking in light of evidence of early-life cognitive and academic abnormalities that this was the only cognitive trajectory subgroup with robust disadvantages in both education and ADHD PGSs, perhaps suggesting a distinct genetic etiology.
The PGS profile for each unaffected sibling subgroup was consistent with the profile in the corresponding schizophrenia subgroup, although only the cognition and education PGSs differed significantly across the sibling subgroups. As with the schizophrenia subgroups, PGS profiles predicted sibling subgroup assignments, and sibling subgroup PGS differences converged with differences in observed academic and cognitive performance. This consistency of affected and unaffected sibling subgroup PGS profiles and associations is further evidence of the importance of inherited polygenic factors in distinguishing the cognitive trajectory subgroups.
Although cognitive impairment is common in schizophrenia, the extent of impairment and the trajectory of development leading to impairment vary considerably. A literature seeking to address this heterogeneity has proposed that developmental trajectory subgroups with distinct patterns of course and outcome can be formed using estimates of premorbid and current intellectual functioning (
17–
21). Recent work has identified brain structure differences across subgroups (
22,
23), but genetic differences have not been examined.
With the completion in recent years of high-quality GWASs for many common disorders and traits, PGSs are becoming accessible research tools. Combined with the increasing availability of large, comprehensively phenotyped and genotyped samples, PGSs have also evolved rapidly as clinical tools, now providing actionable information in conditions such as coronary artery disease (
39). Despite these promising developments, the clinical utility of PGSs in psychiatry is less clear. The variance in diagnostic status explained by any single PGS is not yet adequate to allow biologically informed diagnosis or helpful clinical stratification (
2,
3). Among other factors, comorbidities and pleiotropy in psychiatric disorders and genetic correlations between the disorders and traits such as cognition and education greatly complicate the resolution of genetic influences and risk (
38).
On the other hand, it is exactly these characteristics of psychiatric disorders that may make simultaneous analysis of multiple PGSs a potent strategy for resolving developmental and diagnostic heterogeneity (
40). The present results offer support for this approach. The set of PGSs included diagnosis-based (schizophrenia and ADHD) and trait-based (cognition and education) PGSs, and both contributed to subgroup differentiation. Together, the four PGSs predicted 7.9% of the variance in cognitive trajectory subgroup membership. Although variance explained was relatively modest, employing a set of PGSs was useful in shifting focus beyond simple case-control discrimination toward the prediction of important within-diagnosis differences, and we draw encouragement from the fact that we accounted for within-group variance at a level comparable to that of studies focused on between-group variance (
33,
36). Thus, in ways that parallel recent findings for depression onset (
40), PGS profiles discriminated cognitive developmental trajectories in schizophrenia and were, to a degree, clinically informative, showing associations with facets of illness course and outcome. To be clear, the present findings that four PGSs account for a modest proportion of variance across cognitive development trajectories in schizophrenia do not provide a basis for clinical stratification of new patients or individuals at risk of illness. However, this work illustrates how multiple PGSs might contribute in the future to stratification that has not been achievable in psychiatric disorders with single-diagnosis PGSs.
Various limitations of this study should be considered. The samples are small by the standards of genetics analyses, and the findings await replication (
24). At the same time, while modest in size, the present sample offers a consistent ascertainment approach, comparison samples, and extensive clinical, cognitive, and functional data, as well as genotypes. Moreover, key statistical findings were robust. Another limitation involves the subgrouping approach. Lacking detailed information about developmental history in each case, we employed a proxy measure of “premorbid IQ” as one cornerstone of our subgrouping, as others have done (
17), providing an estimate of cognitive performance in early adolescence, prior to the onset of psychosis. Contrasting characteristics of the subgroups provided some validation of the strategy, but it would be preferable to analyze direct information about pre-diagnosis developmental history (
40).
The use of current methods for creating PGSs also involves certain limitations. Although it is clear that psychiatric disorders involve both common and rare forms of genetic variation, PGSs reflect only common genetic variants, missing important elements of the genetic landscape (
2). Furthermore, PGSs reflect small genetic effects across the genome and offer limited traction for the investigation of specific biological mechanisms. Notably, the various GWASs on which this study’s PGSs were based involved overwhelmingly Caucasian samples. We restricted our analyses accordingly. The strategies employed here may not be available for non-Caucasian samples until high-quality GWASs in such samples are completed.
In conclusion, the findings of this study suggest that adult cognitive data can be used to generate schizophrenia subgroups with distinct trajectories of cognitive development, and that these subgroups are characterized by quite different profiles of psychiatric, cognitive, and academic genetic influence.