Rising rates of suicide constitute a major threat to public health worldwide. The latest national epidemiological survey of U.S. adults showed a recent overall increase in suicide attempts among those with less formal education and those with depressive and anxiety disorders, among other groups (
1). In addition to imposing an enormous emotional burden on families and communities, suicide attempts carry a high risk of mortality (
2) and often lead to high costs, both direct and indirect (
3). Faced with this increasing burden of suicidal behavior, researchers have redoubled their efforts to elucidate risk factors and preventive strategies for suicide.
Genetic contributions to suicidal behavior have long been suspected on the basis of family, twin, and adoption studies (reviewed in reference
4). However, genome-wide association studies (GWASs), which have successfully detected numerous genetic markers for a variety of psychiatric and other common illnesses, have until now failed to find statistically significant markers for suicide. In this issue of the
Journal, Mullins et al. (
5) report the results of a large-scale GWAS of suicidal behavior—the largest to date—based on several samples contributed to the Psychiatric Genomics Consortium. The researchers compared genetic marker data obtained from people diagnosed with major depressive disorder, bipolar disorder, or schizophrenia who reported suicide attempts to data from people with the same diagnoses who denied any history of suicidal behavior. This design controls for the fact that almost everyone who engages in suicidal behavior suffers from a mental illness. A meta-analysis was then conducted across all three diagnostic groups, enhancing the opportunities to detect cross-diagnostic markers of suicidal behavior. Finally, the authors used the polygenic risk score (PRS) approach to investigate whether genetic liability to depression, bipolar disorder, or schizophrenia per se was associated with risk of suicide attempts.
Key Findings
The results represent an important step forward in the genetics of suicidal behavior. In people with bipolar disorder, suicide attempts were associated in a small but significant way with a common genetic marker on chromosome 4. Although this marker does not immediately implicate any particular genes, the marker also reached genome-wide significance in the meta-analysis of suicide attempts in mood disorders, suggesting that it is picking up something that bipolar disorder and major depression have in common, perhaps depressive symptoms. The marker was not replicated in the two large independent mood disorder cohorts tested, however, so replication is advisable before pursuing this region on a molecular basis.
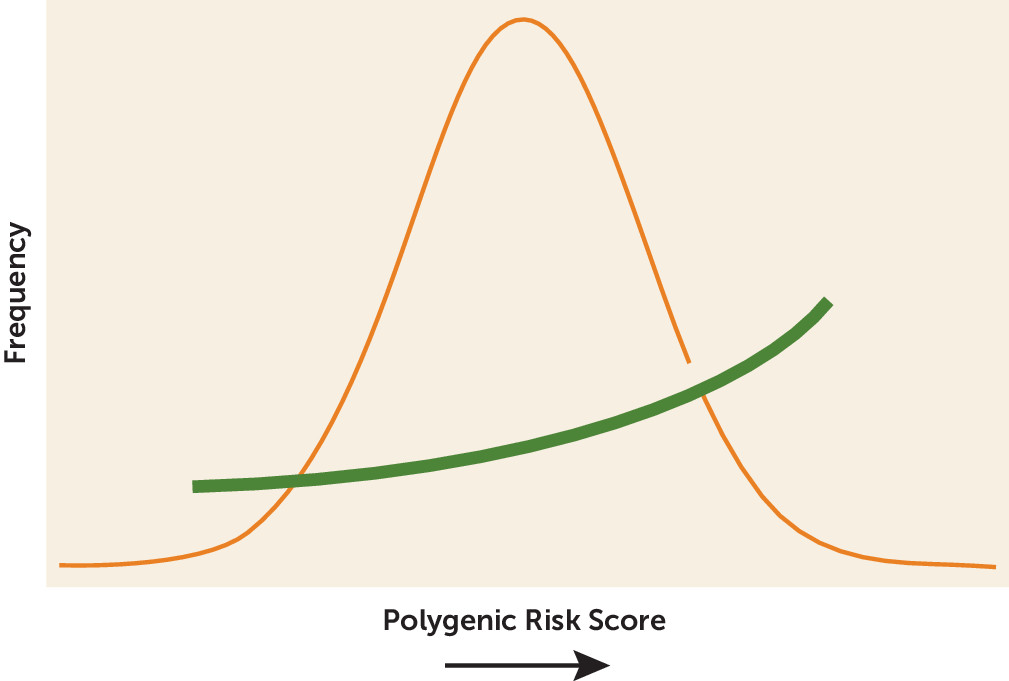
The PRS results were especially intriguing. PRS adds up the risk conferred by thousands of genetic markers across the whole genome (
Figure 1), so it can account for the large number of small genetic effects typically seen in complex disorders (
6). PRS is especially useful when complex traits are determined by variations that have small effect sizes, occur in many genes, and interact with environmental factors over a long period of time (
7). Here the PRS analysis showed that genetic risk for major depression increases risk for suicide attempts in people with major depression, as might be expected. However, the results showed that genetic risk for major depression also increased risk for suicide in people diagnosed with bipolar disorder or schizophrenia. Genetic risk for bipolar disorder or schizophrenia showed no such association with suicide attempts. As Mullins et al. note: “This study is the first to show that across disorders, suicide attempters carry a greater burden of depression risk alleles rather than simply a higher genetic liability for the psychiatric disorder they are affected by.” This finding is also a reminder that everyone carries a blended mix of many different risk alleles for many different disorders and that one genetically influenced trait—here suicidal behavior—may become apparent in the setting of another disorder.
Limitations
As the largest suicide GWAS to date, this study provides a solid starting point and some valuable clues about the underlying genetic architecture of suicide, but some important limitations should be kept in mind. First, the scoring of suicide attempt and non-attempt was based on various clinical assessments, with variable definitions of what constitutes a suicide attempt, and strong reliance on self-report, which may underestimate suicidal behavior. This heterogeneous case definition may help explain the lack of replication for the top GWAS hit. Second, this study, like most in the literature, only includes people who survived a suicide attempt, thus excluding the more than 50% of suicidal patients who die in their first attempt (
2) and may well represent those at highest genetic risk. Finally, while this study used lifetime suicide attempts as the main trait, other clinical variables, such as recurrence (
8), use of guns or other violent means of death, and planned versus impulsive attempts, may help define important clinical and genetic subtypes with greater shared genetics and biology (
4).
One big question raised by studies like this has to do with what a “depression polygenic score” really represents. There is a very wide range of people who may be included as “cases” in a genetic study of depression, ranging from those with recurrent, severe, psychotic major depression to those who simply endorse some depressive symptoms on a questionnaire. Most of the genetic risk factors for depression that have been found so far come from GWASs based largely on less severe, nonclinical samples. Comparison with other GWASs shows strong genetic overlaps with anxiety, neuroticism, smoking, and general help-seeking, which may be quite different from what most clinicians would diagnose as major depressive disorder (
9,
10). Significant genetic correlations between suicide attempts and depressive symptoms, major depression, neuroticism, and disordered sleep traits have also been reported (
11). Taken together, these findings suggest that a “depression polygenic score” actually reflects a very broad range of psychopathology, far beyond DSM definitions of major depression. This may be one reason only the “depression” PRS was associated with suicidal behavior across diagnoses. This has important clinical implications, suggesting that we need to take a wider view of who is at risk for suicide so that primary prevention can be undertaken. It also suggests that untangling the shared genetics of depression, anxiety, neuroticism, and other common ills will be very challenging.
PRS has gained popularity in many fields of medicine because there is hope that as information from GWASs accumulates, PRS could play a role in identifying people at high risk for a given disease or even assist with diagnosis (
7,
12). Right now, however, we are nowhere near the point where we can use PRS in the psychiatric clinic. Even the strongest predictions we have so far (based on GWAS of schizophrenia) show that more than 80% of people with a high schizophrenia PRS have not been diagnosed with schizophrenia (
13). In general, a low PRS is no guarantee that disease will not occur, and even for a person with a high PRS, both rare genetic variants (not counted in the PRS) and nongenetic factors—including therapeutic interventions—clearly play important roles in risk and resilience. While evidence from the cardiovascular literature suggests that individuals with a high PRS for heart disease are at clinically significant risk for myocardial infarction and may benefit from early prevention (
14), further research, including much larger GWASs with longitudinal follow-up, will be needed to determine whether PRS will have any such clinical utility in psychiatric care.